Lewis dot structure for cocl2
By now, you have probably noticed a pattern in covalent bond formation. When atoms form the normal number of covalent bonds with other elements, they may do so in any manner that sums to equal the normal number of bonds. This means it has four valence electrons and normally makes four covalent bonds. As a result, when carbon bonds with other elements, all of the following bond combinations are possible:, lewis dot structure for cocl2.
Name the third and fourth transition elements of first transition series. What is the coordinate number of the central metal ions in the following coordination compound? The resistance of a 0. Calculate the molar conductivity of the solution if the electrods in the cell are 1. Draw the Lewis dot structures for sulphurtrioxide.
Lewis dot structure for cocl2
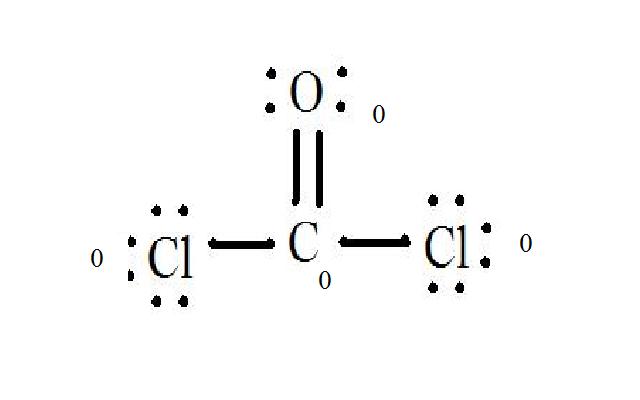
When determining the formal charge of a molecule such as CoCl2 phosgene gas , you need to know the number of valence electrons for each atom and the Lewis structure of the molecule. Look up each atom in the periodic table of elements to determine the number of valence electrons. Remember that two electrons go in the first s shell, two electrons in the second s shell, six electrons in the first p shell, etc. Adjust for charge. If the molecule is an ion, add or subtract one or more electrons overall to account for the final charge. The molecule is not ionized and has a neutral charge. See the diagram for the Lewis structure of CoCl2 phosgene gas. The Lewis structure represents the most stable and probable structure for a molecule. Atoms are drawn with paired valence electrons; bonds are formed between lone electrons to satisfy the octet rule. The chloride atoms share single bonds with the carbon molecule, while the oxygen atom forms a double bond with carbon. Each atom in the final structure satisfies the octet rule and has eight valence electrons allowing for molecular stability. Count the lone pairs of each atom in the Lewis structure.
The only radioactive element among lewis dot structure for cocl2 lanthanoids is. Notice that the structure on the left has 3 F atoms attached to one C atom and 3 H atoms attached to the other C atom. Atoms are drawn with paired valence electrons; bonds are formed between lone electrons to satisfy the octet rule.
Carbon, in group 4 or 14, has 4 valence electrons. Oxygen in group 6, sometimes called 16, 6 valence electrons. Seven for Chlorine but we have two Chlorines. Add it all up, 4 plus 6 plus 14, you have a total of 24 valence electrons. Carbon is the least electronegative. We'll put that in the center. Put the Oxygen and then the two Chlorines around the outside.
Ready to learn how to draw the lewis structure of COCl2? Here, I have explained 6 simple steps to draw the lewis dot structure of COCl2 along with images. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of COCl2. Here, the given molecule is COCl2.
Lewis dot structure for cocl2
COCl 2 phosgene has one carbon atom, one oxygen atom, and two chlorine atoms. In the COCl 2 Lewis structure, there are two single bonds and one double bond around the carbon atom, with two chlorine atoms and one oxygen atom attached to it. Two chlorine atoms with single bonds have three lone pairs, and one oxygen atom with a single bond has two lone pairs. In the periodic table , carbon lies in group 14, oxygen lies in group 16, and chlorine lies in group Hence, carbon has four valence electrons, oxygen has six valence electrons, and chlorine has seven valence electrons. Learn how to find: Carbon valence electrons , Oxygen valence electrons , and Chlorine valence electrons. We have a total of 24 valence electrons. And when we divide this value by two, we get the value of total electron pairs. Since carbon is less electronegative than oxygen and chlorine, assume that the central atom is carbon.
Huge macrame wall hanging
Draw lewis dot structure of phosphoric acid H 3 P O 4. Final check: The total number of electrons shown in the molecule must equal the number of valence electrons calculated in the first step. Carbon, in group 4 or 14, has 4 valence electrons. Add electrons as pairs to make an octet around each atom in the molecule. They are isomers of each other. You'll need to form a double bond between the Carbon and Oxygen to complete the octet on the Carbon atom. The bivalent metal ion having maximum paramagnetic bahaviour among the Draw the lewis dot structure of COCl2. Sign in. The resistance of a 0. Updated April 24, But in the center, Carbon only has 6 valence electrons. Look up each atom in the periodic table of elements to determine the number of valence electrons. Non Neutral Atoms Examples.
Phosgene is a colorless gaseous compound known as carbonyl chloride and has a molecular weight of It is non-flammable in nature and bears a suffocating odor. It has a boiling point b.
Carbon is the least electronegative. Each F atom is making 1 bond, as expected. Calculating the formal charge for molecules containing transition metals can be tricky. Draw lewis dot structure of C H 4. Opens New Window. Draw the lewis dot structure of CCl4. Number of valence electrons:. If necessary, use double bonds or triple bonds to achieve the normal number of covalent bonds for each atom. What is the coordinate number of the central metal ions in the following coordination compound? Choose the correct matching of transition metal ion and magnetic momen Every atom in the molecule makes the expected number of bonds. How would you account for the increasing oxidising power in the series The correct electronic configu

You are not right. I can prove it. Write to me in PM, we will talk.