Lewis dot structure for chcl3
Get a free answer to a quick problem. Most questions answered within 4 hours. Choose an expert and meet online. No packages or subscriptions, pay only for the time you need.
Lewis structures are used to describe and visualize molecules. Lewis structures are used to show the bonds between atoms as well as the electrons surrounding certain atoms. Note: The periodic table shows you how many valence electron each element has. Sometimes, we need to visualize a molecule. To help our visualization, we draw a Lewis dot structure. When we draw lewis dot structures, we are looking at covalently bonded molecules. The atoms are sharing pairs of electrons to fill their octets.
Lewis dot structure for chcl3
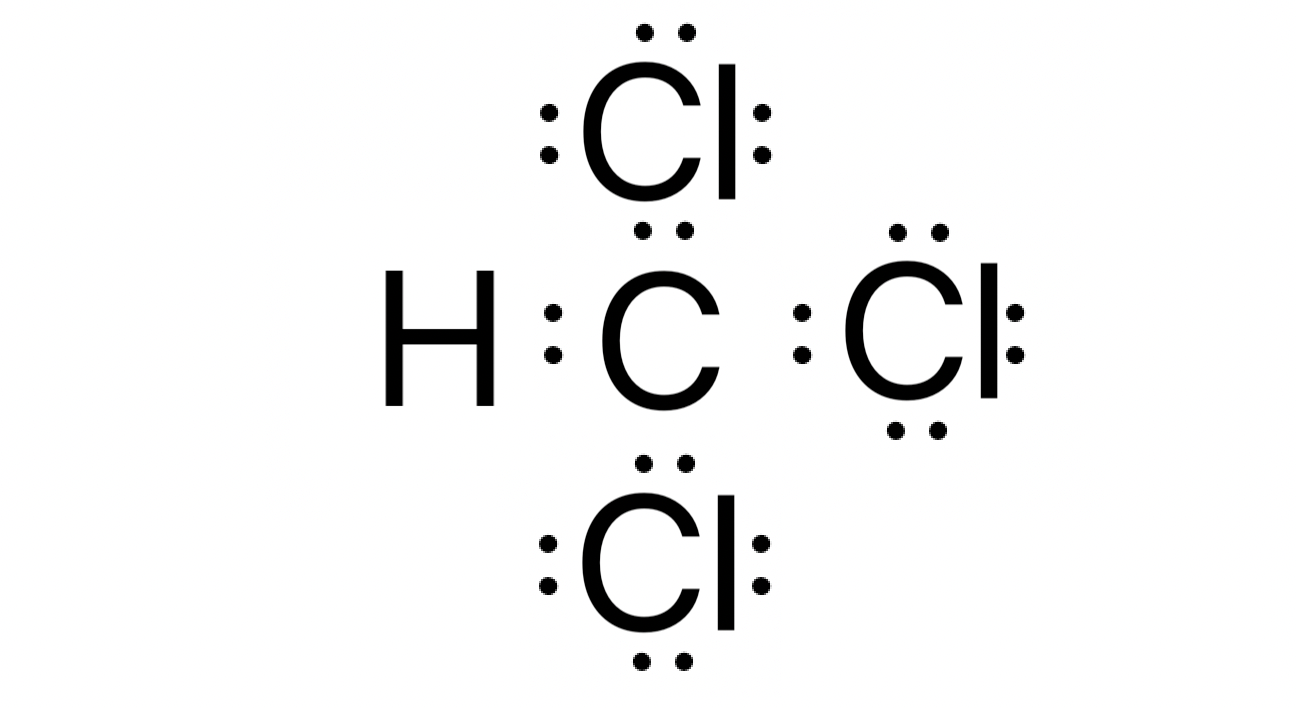
Chloroform CHCl 3 contains one carbon atom, three chlorine atoms and one hydrogen atom. In the lewis structure of CHCl 3 , carbon atom is located as the center atom and other atoms have made bonds with carbon atom. There are three chlorine atoms around center carbon atom. Hydrogen atom has made a single bond with carbon atom and each chlorine atom has three lone pairs on their valence shell. As well, there are no charges on atoms in CHCl 3 lewis structure. When we draw a lewis structure, there are several guidelines to follow. Number of steps can be changed according the complexity of the molecule or ion. However those all steps are mentioned and explained in detail in this tutorial for your knowledge. There are three elements in chloroform; carbon, hydrogen and chlorine. Hydrogen is a group IA element in the periodic table and only has one electron in its last shell valence shell. Carbon is a group IVA element in the periodic table and has four electrons in its last shell valence shell. Also, Chlorine is a group VIIA element in the periodic table and contains seven electrons in its last shell. Now, we know how many electrons are there in valence shells of hydrogen, carbon and chlorine atoms.
Molecules with an odd number of electrons have a single electron dot instead of a lone pair. Carbon's four single bonds give it eight electrons. Leave a half-inch gap between the central atom and the surrounding elements.
.
The chemical formula CHCl3 represents Chloroform. It is also known as Trichloromethane. Chloroform is a clear, colorless liquid that possesses a pleasant odor. It is nonflammable and is denser than water. It is generally prepared by the chlorination of methane. Chloroform first found use as an inhalation anesthetic in the 19th century. These days, it is produced industrially as a precursor to making Teflon.
Lewis dot structure for chcl3
In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. In this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely Lewis symbols and Lewis structures. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons:. Figure 7. Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium:. Likewise, they can be used to show the formation of anions from atoms, as shown here for chlorine and sulfur:. We also use Lewis symbols to indicate the formation of covalent bonds, which are shown in Lewis structures , drawings that describe the bonding in molecules and polyatomic ions.
Silicone cupping therapy massage cups
What are lewis structures? Minimize the total number of formal charges. So, it only forms three bonds. Sulfur is the central atom. The lewis structure of sulfur dioxide has sulfur double bonded to both oxygens. For hypovalent molecules, the central atom does not have to obey the octet rule. Write a capital C. The twelve remaining electrons will be lone pair dots around the oxygens. We should memorize which elements can have more than eight electrons. Because three are no charges on atoms, we do not need to do the step of reducing charges on atoms by converting lone pairs to bonds. You can find the number of valence electrons by looking at each atom's atomic number on the periodic table. The other oxygen should have a double bond to sulfur and two lone pairs.
CHCl 3 chloroform has one carbon atom, one hydrogen atom, and three chlorine atoms. In the CHCl 3 Lewis structure, there are four single bonds around the carbon atom, with one hydrogen atom and three chlorine atoms attached to it, and on each chlorine atom, there are three lone pairs. In the periodic table , carbon lies in group 14, hydrogen lies in group 1, and chlorine lies in group
In this case, Sulfur has only 6 electrons, so it needs 2 more electrons to complete its octet, thus we will form a double bond between one of the oxygens to complete its octet! Because, carbon can show higher valance than oxygen, carbon has the higher chance to be the center atom in CHCl 3. The sulfur has a positive formal charge. The sulfur has a positive one formal charge. The carbon will have zero lone pairs. We should memorize which elements can have more than eight electrons. Now, we know how many electrons are there in valence shells of hydrogen, carbon and chlorine atoms. One oxygen should have a single bond to sulfur and three lone pairs. The other oxygen should have a double bond to sulfur and two lone pairs. Here are some tips for the octet rule exceptions.

Between us speaking, in my opinion, it is obvious. Try to look for the answer to your question in google.com
Willingly I accept. An interesting theme, I will take part. I know, that together we can come to a right answer.
You are not right. I am assured. I can prove it. Write to me in PM, we will discuss.