Lewis dot of h2s
Draw the electron dot structures for a ethanoic acid b H2S c propanone d F2- Find the answer to this question and access a vast question bank that is customised for students.
Hydrogen sulfide H2S consists of two hydrogen H atoms and one sulfur S atom. Sulfur is located in group 16 of the periodic table, indicating that it has six valence electrons, while hydrogen belongs to group 1 and brings one valence electron per atom. Determining the Total Valence Electrons. To accurately represent the H2S Lewis structure, we need to calculate the total valence electrons. Sum the valence electrons of each atom:. Identify the Central Atom.
Lewis dot of h2s
There are 2 single bonds between the Sulfur atom S and each Hydrogen atom H. There are 2 lone pairs on the Sulfur atom S. In order to find the total valence electrons in H2S molecule , first of all you should know the valence electrons present in hydrogen atom as well as sulfur atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. Sulfur is a group 16 element on the periodic table. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Remember: If hydrogen is present in the given molecule, then always put hydrogen outside. Now here the given molecule is H2S dihydrogen sulfide and it contains hydrogen atoms H and sulfur atom S. You can see the electronegativity values of hydrogen atom H and sulfur atom S in the above periodic table.
In Indian rupees, 1 trillion is equal to how many crores?
The Lewis structure of H2S consists of a central sulphur atom S and two external hydrogen atoms H at a The sulphur atom S and the two hydrogen atoms H are each connected by a single bond. The Lewis structure of H2S is shown below:. Sulphur and hydrogen are elements of group 16 and group 1 of the periodic table, respectively. Therefore, there are 6 valence electrons in a sulphur atom and 1 valence electron in a hydrogen atom.
The hydrogen sulfide chemical formula is H2S. Drawing H2S Lewis Structure is very easy to by using the following method. Here in this post, we described step by step method to construct H2S Lewis Structure. The sulfur and hydrogen elements come as the member of the oxygen and hydrogen family groups from the periodic table respectively. The valence electrons in sulfur and hydrogen are six and one respectively. Hydrogen sulfide is used to make chemical reagents for organic chemical reactions for the production of sulfur-organic materials. A three-step approach for drawing the H2S Lewis structure can be used. The first step is to sketch the Lewis structure of the H2S molecule, to add valence electrons around the sulfur atom; the second step is to add valence electrons to the two hydrogen atoms, and the final step is to combine the step1 and step2 to get the H2S Lewis Structure.
Lewis dot of h2s
Also, there are two lone pairs around sulfur atom. Concept of number of total valence electrons of sulfur and hydrogen atoms are used to draw lewis structure of H 2 S. Each step of drawing lewis structure of H 2 S is explained in detail in this tutorial. Lewis structure of H 2 S is similar to the lewis structure of H 2 O. There are two S-H bonds and two lone pairs around sulfur atom in H 2 S lewis structure. There are several steps to draw the lewis structure of H 2 S. Those steps are explained in detail in this tutorial. Because H 2 S molecule is a simple molecule, some of these steps are not used. In such cases, they are mentioned with those respective steps.
Katwa restaurant
Based on formal charges and octet rule considerations, fine-tune the electron placement until an optimal Lewis structure is achieved. The central sulfur S atom in H2S undergoes sp3 hybridization, forming four hybrid orbitals for bonding and lone pairs. In short, now you have to find the formal charge on hydrogen H atoms as well as sulfur S atom present in the H2S molecule. The sulphur atom S and the two hydrogen atoms H are each connected by a single bond. An octet rule governs Lewis structures. In the Lewis structure of hydrogen sulphide, the sulphur atom has two lone pairs of electrons and two bonded pairs of electrons, there are eight electrons which form an octet and therefore the sulphur atom is stable. This indicates that the above lewis structure of H2S is stable and there is no further change in the above structure of H2S. Check Octet Rule and Adjust. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. It is an essential component for energy production in cells Table of Content. The Lewis structure of H2S consists of a central sulphur atom S and two external hydrogen atoms H at a Each hydrogen H atom contributes a single electron to form a covalent bond with sulfur. The Lewis structure of H 2 S is based on the number of total valence electrons present in sulphur and hydrogen atoms. H2S Lewis Structure.
Hydrogen Sulfide is a common chemical compound that is useful for analyzing inorganic compounds of metal ions. It has the chemical formula of H 2 S. The molecule has two Hydrogen atoms and a single Sulfur atom.
The lone pair of electrons occupies two sp3 orbitals. To accurately represent the H2S Lewis structure, we need to calculate the total valence electrons. Sulfur is a group 16 element on the periodic table. It is poisonous and has a foul odor like a rotten egg. It is an essential component for energy production in cells So you have seen the above image by now, right? The stability of lewis structure can be checked by using a concept of formal charge. Draw the Electron Dot Structures for Draw the electron dot structures for a ethanoic acid b H2S c propanone d F2- Find the answer to this question and access a vast question bank that is customised for students. Remember : If hydrogen is present in the molecule, always place the hydrogen atoms on the outside. H2S does not exhibit resonance structures since there are no multiple bond placements or delocalized electrons within the molecule. The Lewis structure of H2S consists of a central sulphur atom S and two external hydrogen atoms H at a Valence electrons are the electrons that are present in the outermost orbit of any atom.

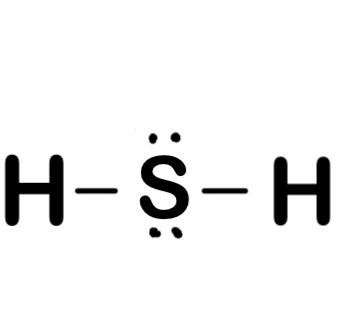
Just that is necessary, I will participate.
It is a pity, that now I can not express - I am late for a meeting. But I will be released - I will necessarily write that I think.
Good topic