Lewis dot diagram for mg
This chapter will explore yet another shorthand method of representing the valence electrons.
File Talk. Read View on Commons. Tools Tools. This is a file from the Wikimedia Commons. Information from its description page there is shown below. Commons is a freely licensed media file repository. You can help.
Lewis dot diagram for mg
.
Valence electrons are the electrons responsible for chemical reactions. Write the Lewis electron dot formula for: a Oxygen b Sulfur c Potassium d Carbon Lewis dot diagram for mg : a Oxygen has the electron configuration: 1 s 2 2 s 2 2 p 4therefore there are 2 core electrons and 6 valence electrons.
.
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot diagram or electron dot diagram, or a Lewis diagram, or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side.
Lewis dot diagram for mg
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot diagram or electron dot diagram or a Lewis diagram or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. It does not matter what order the positions are used. For example, the Lewis electron dot diagram for hydrogen is simply:. Because the side is not important, the Lewis electron dot diagram could also be drawn as follows:.
Lojel luggage australia
One way to represent this valence electron, visually, was developed by Gilbert N. Captions English Add a one-line explanation of what this file represents. As defined earlier in this chapter, core electrons are all the electrons except the valence electrons and valence electrons are the electrons in the outermost energy level. This is a file from the Wikimedia Commons. Just as the electrons are placed singly before being doubled up in the orbital representation, so they are placed one at a time in the p orbitals of an electron dot formula. Tools Tools. Summary Description Lewis dot Mg. Look at Table 9. The Lewis electron dot formula is: b Sulfur has the electron configuration: 1 s 2 2 s 2 2 p 6 3 s 2 3 p 4 , therefore there are 10 core electrons and 6 valence electrons. The electron configuration for chlorine could be written as: 1 s 2 2 s 2 2 p 6 3 s 2 3 p 5. Policies and guidelines Contact us.
This chapter will explore yet another shorthand method of representing the valence electrons.
Notice that the electrons are in groups of two. Lewis suggested that since the valence electrons are responsible for chemical reactions and the core electrons are not involved, we should use a diagram that shows just the valence electrons for an atom. Then we place the valence electrons around the sides of the box with each side representing an orbital in the outermost energy level. Captions English Add a one-line explanation of what this file represents. There are 2 valence electrons, 3 s 2. We will, as we observed in the previous lesson, finish the lesson with a look at how this visual representation flows in a pattern throughout the Periodic Table. Valence electrons are the electrons responsible for chemical reactions. Reading room forum Community portal Bulletin Board Help out! One way to represent this valence electron, visually, was developed by Gilbert N. Policies and guidelines Contact us. The electron configuration is: 1 s 2 2 s 2 2 p 6 3 s 1. Think of the chlorine in a box and the box has 4 sides. Look at Table 9.

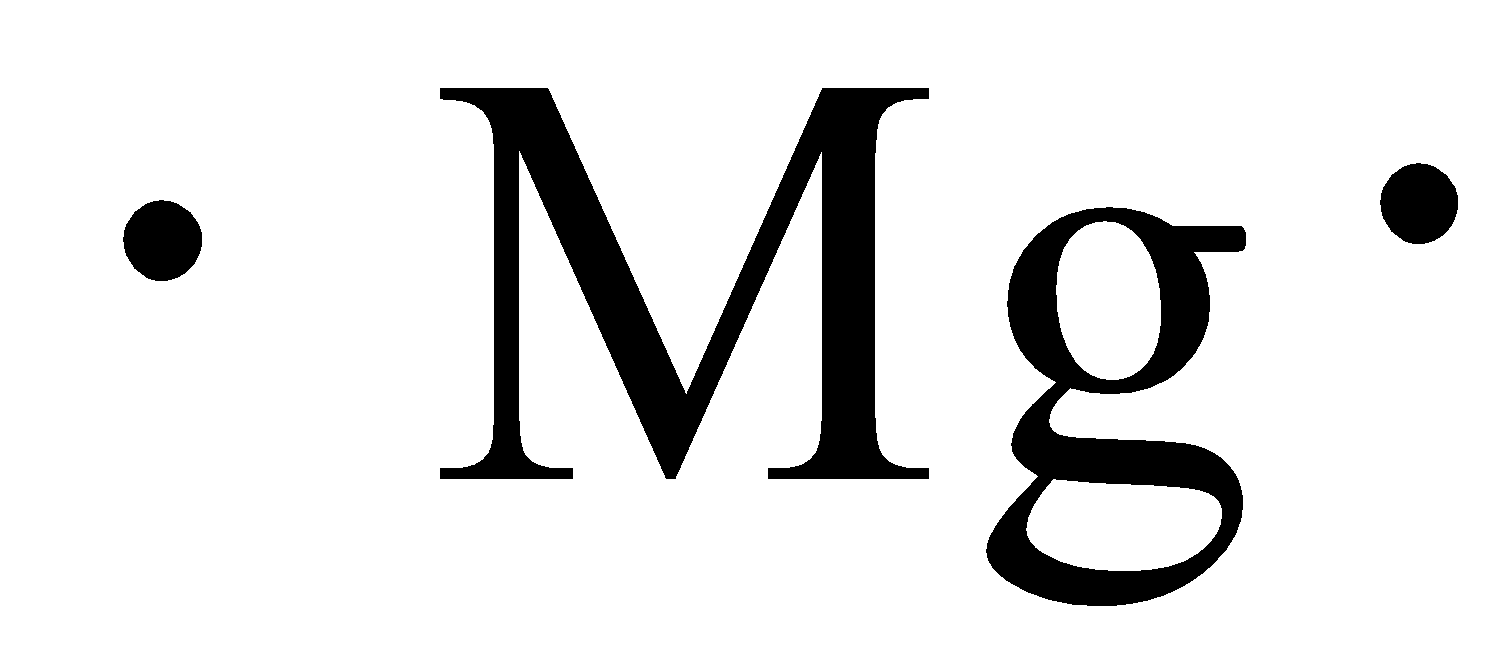
0 thoughts on “Lewis dot diagram for mg”