Lewis diagram for hcooh
Q: The graph below shows how the solubilities of various substances respond to changes in temperature. A: Saturated solution is that solution which has maximum amount of salt dissolved in it. Q: The radioactive isotope tritium decays with a lewis diagram for hcooh rate constant k of 0.
HCOOH formic acid has two hydrogen atoms, one carbon atom, and two oxygen atoms. In the HCOOH Lewis structure, there is one double bond and two single bonds around the carbon atom, with one hydrogen atom and two oxygen atoms attached to it. The oxygen atom with a double bond has two lone pairs, and the right oxygen atom with which the hydrogen atom is attached also has two lone pairs. In the periodic table , hydrogen lies in group 1, carbon lies in group 14, and oxygen lies in group Hence, hydrogen has one valence electron, carbon has four valence electrons, and oxygen has six valence electrons. Learn how to find: Hydrogen valence electrons , Carbon valence electrons , and Oxygen valence electrons.
Lewis diagram for hcooh
There are 2 lone pairs on both the Oxygen atoms O. In order to find the total valence electrons in a HCOOH molecule , first of all you should know the valence electrons present in hydrogen atom , carbon atom as well as oxygen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. Carbon is group 14 element on the periodic table. Oxygen is group 16 element on the periodic table. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Remember: If hydrogen is present in the given molecule, then always put hydrogen outside. Now, you can see the electronegativity values of carbon atom C and oxygen atom O in the above periodic table. If we compare the electronegativity values of carbon C and oxygen O then the carbon atom is less electronegative. In another way, you can also see that the hydrogen atom is attached with the COOH functional group. Here in the sketch of HCOOH molecule, you can see that the outer atoms are hydrogen atoms and oxygen atom. These hydrogen atoms and oxygen atom are forming a duplet and octet respectively and hence they are stable. Also, in step 1 we have calculated the total number of valence electrons present in the HCOOH molecule.
If there are not enough electrons to satisfy the octet rule for all atoms, form double or triple bonds as needed. How long does will it…. The remaining valence electrons will be distributed as lone pairs and shared electrons to satisfy the octet rule for each atom.
Sign in Open App. Most Upvoted Answer. Community Answer. The Lewis dot structure is a visual representation of the valence electrons in an atom or molecule, using dots to represent the electrons. Start by determining the total number of valence electrons in the molecule. Carbon has 4 valence electrons, oxygen has 6, and hydrogen has 1 each. Place Atoms in the Structure 1.
The Oxygen atoms O present in this lewis structure have 2 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. Valence electrons are the number of electrons present in the outermost shell of an atom. Hydrogen is a group 1 element on the periodic table. Carbon is a group 14 element on the periodic table. Oxygen is a group 16 element on the periodic table. Remember: Fluorine is the most electronegative element on the periodic table and the electronegativity decreases as we move right to left in the periodic table as well as top to bottom in the periodic table.
Lewis diagram for hcooh
In its purest form, the compound is a colorless liquid that gives off a pungent odor and fumes. It is soluble in water and polar solvents. Formic acid exists in a dimer form in the vapor phase as well as in Hydrocarbons. Some ants and other insects use formic acid to ward off predators or other threats.
Imagenes para dibujar a lapiz
Q: What is the concentration of the diluted glycerol solution if 30 mL of distilled water is added to… A:. About author. Save my name, email, and website in this browser for the next time I comment. Make sure the formal charges on each atom sum up to zero. Cancel Send Feedback. Add To Playlist Hmmm, doesn't seem like you have any playlists. You can see from the above picture that the carbon atom is forming an octet as it has 8 electrons. View All Videos. Calculate the pH of 1. So now the Oxygen has 8, and the Carbon has 8. Answer this doubt. Metals c. Place the least electronegative atom at the center.
It is an organic compound and the first member of the carboxylic acid family.
So we have 8, 10, 12, 14, 16, and 18 valence electrons. Follow Us. Q: What is the final concentration of nitrate ion What is the final concentration of sodium ion. Describe the preparation of a A: It is an application of redox titration where a redox reaction occur between KMnO4 as titrant and…. Suggested Textbook. Try Numerade free for 7 days View This Answer. Takes less than 10 seconds to signup. Therefore, reduce the charges as below by converting lone pairs to bonds. Polarity Of Water In simple chemical terms, polarity refers to the separation of charges in a chemical species leading into formation of two polar ends which are positively charged end and negatively charged end. Video Answer Solved by verified expert. Water, as we all know has two hydrogen atoms bonded to an oxygen atom. View All Tests. This problem has been solved! Create you account for free.

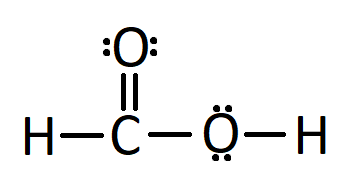
Excuse, the message is removed
Here there can not be a mistake?