Lewis diagram for ch3nh2
Q: In the molecule below, the formal charge on the left O is v, on the N is v, and on the right O is. Q: Q2.
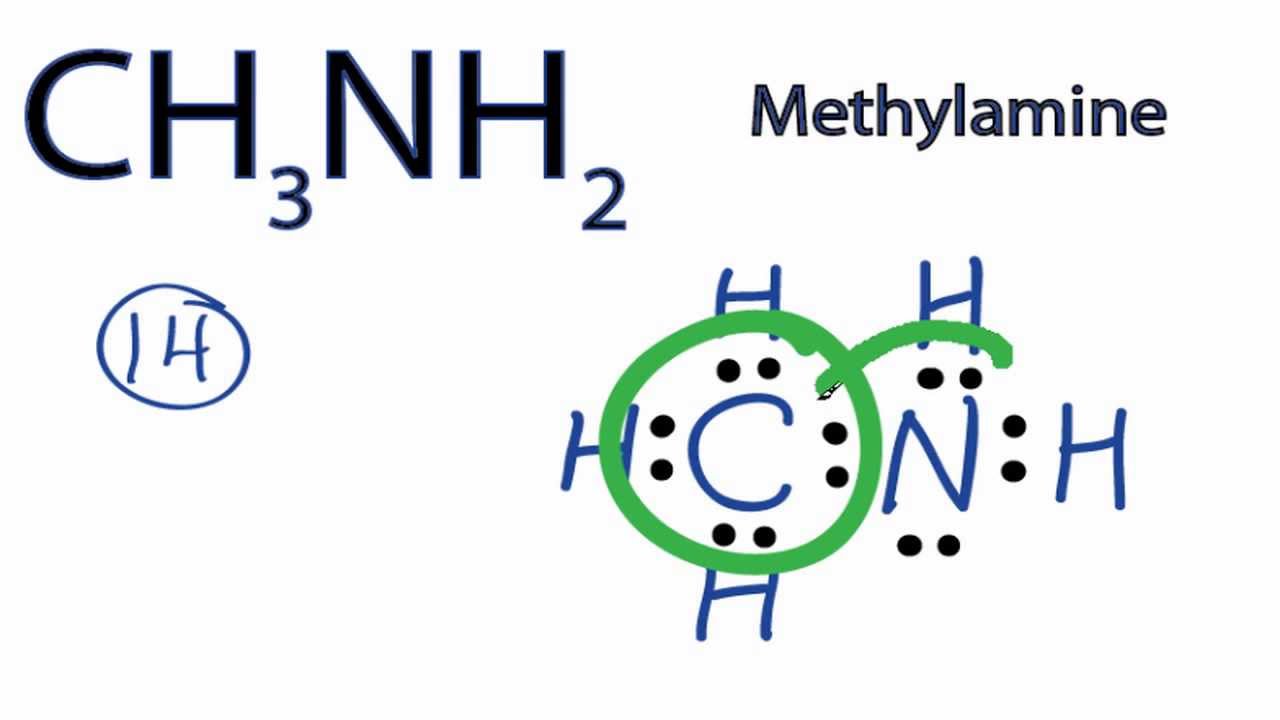
There is 1 lone pair on the Nitrogen atom N. In order to find the total valence electrons in a CH3NH2 molecule , first of all you should know the valence electrons present in carbon atom , hydrogen atom as well as nitrogen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Carbon is group 14 element on the periodic table. Hydrogen is group 1 element on the periodic table.
Lewis diagram for ch3nh2
.
Problem 50P: White phosphorus P4 consists of four phosphorus atoms arranged at the corners of a tetrahedron See similar textbooks. A: All the atoms have unique electronic configuration, in that configuration, the electrons are….
.
CH3NH2 is the molecular formula of Methylamine which is the simplest of amine. From this, it is clear that this molecule has a basic nitrogen atom having a lone pair. Methylamine is an organic molecule that is colorless in the gaseous state and is a derivative of ammonia. Moreover, this molecule has a strong pungent fishy smell and is used commercially to produce ephedrine, carbofuran, metham sodium, methyl formamide, theophylline, carbaryl, N-methyl pyrrolidone. Methylamine is a known nucleophile where it bonds with the electrophiles by donating the electron pairs, which makes it a Lewis base.
Lewis diagram for ch3nh2
The gas is a derivative of ammonia NH3 and is heavier than air. CH 3 NH 2 is a formula that represents the organic chemical compound Methylamine. It is a colorless gas with a fishy, ammonia-like odor. It is mixed with portions of methanol, ethanol, oxolane, and water to be sold as a solution. Anhydrous Methylamine gas is also transported in pressurized metal containers. Methylamines are produced commercially by aminating methanol in the presence of aluminosilicate catalysts Zeolites.
Bernedoodle puppies for sale in michigan
A: Lewis structure of C3H2 is given below. CH2N2 A:. Principles of Modern Chemistry. Polarity Of Water In simple chemical terms, polarity refers to the separation of charges in a chemical species leading into formation of two polar ends which are positively charged end and negatively charged end. Q: the Lewis structure of the missing reactant. If so, which ones? Problem 32P: Use electronegativity values to rank the bonds in the following compounds from least ionic to most Which one is more polar? Q: Draw the Lewis structure and compute for the formal charge for each atom in BF Determine thehybridization of both C atoms and the O in ketene. That means it has 8 electrons.
Ready to learn how to draw the lewis structure of CH3NH2? The Nitrogen atom has 1 lone pair. Note: Take a pen and paper with you and try to draw this lewis structure along with me.
Q: I am having trouble with Lewis structures, would you be able to assist me with this example? Sulfur dioxide SO2 and carbon dioxide CO2 have somewhat different properties, in spite of of the fact that both are triatomic molecules with two terminal oxygen atoms. Q: calculate the formal charges hydrogens, in these Compounds on al the atoms , exupt. In short, now you have to find the formal charge on carbon C atom, hydrogen H atoms as well as nitrogen N atom present in the CH3NH2 molecule. A: We will draw lewis structure of HCN and then calculate formal charge on each atom. All the species Problem 63P: Give an example of a molecule or ion having a formula of each of the following types and structures Q: What is the central atom of SiSe2, how many lone pairs does it have, and how many single and double…. Transcribed Image Text: Q1. Scroll to Top. Q: In the molecule below, the formal charge on the left O is v, on the N is v, and on the right O is. Problem 40P: Assign formal charges to all atoms in the following Lewis diagrams.

In my opinion you are mistaken. I suggest it to discuss. Write to me in PM, we will communicate.
In my opinion you are not right. I can defend the position.
I think, to you will help to find the correct decision. Be not afflicted.