Lewis diagram for c2h6
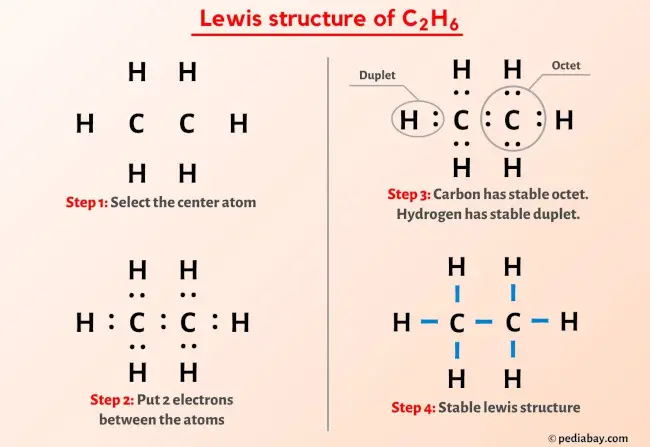
C 2 H 6 ethane has two carbon atoms and six hydrogen atoms, lewis diagram for c2h6. In the C 2 H 6 Lewis structure, there is a single bond between the two carbon atoms, and each carbon is attached with three hydrogen atoms, and none of the atoms has a lone pair.
Since C has 4 valence electrons , and each H atoms contributes 1 valence electron, the total number of electrons will be. So, the two C atoms are placed in the center of the molecule. Each C atom forms three covalent bonds with three H atoms, with one aditional covalent bond being formed between the two C atoms. Each of these seven single bonds contains 2 electrons, which means that a total of. How can I write the Lewis dot structure for C2H6? Stefan V. Jan 11,
Lewis diagram for c2h6
Carbon is the central atom, hydrogen is the outer atom, there is a single bond between the two carbon atoms, each carbon atom is connected to three hydrogen atoms by a single bond, and none of the atoms have a lone pair of electrons. The C2H6 Lewis structure is shown below:. Carbon and hydrogen are group 14 and group 1 elements in the periodic table. The central atom must satisfy the principle of less electronegativity. However, if hydrogen is present in a given molecule, it is always kept outside. So for the C2H6 or ethane molecule, even though the hydrogen atoms are less electronegative than the carbon atoms, we must leave the hydrogen on the outside. Thus, the carbon atom C is the central atom and the hydrogen atom H is the outer atom. For the C2H6 molecule, the total number of pairs of electrons is seven. In Step 3 we can see that the external hydrogen atoms in the C2H6 molecule are forming a bimolecule, so they are stable. In addition, we must check that the central carbon atom C is stable, and we can see from the above steps that both carbon atoms are forming an octet. This means that they have 8 electrons.
Thus, C2H6 is sp3 hybridized. The stability of lewis structure can be checked by using a concept of formal charge.
In order to find the total valence electrons in C2H6 molecule , first of all you should know the valence electrons present in carbon atom as well as hydrogen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Carbon is group 14 element on the periodic table. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Remember: If hydrogen is present in the given molecule, then always put hydrogen outside.
C 2 H 6 ethane has two carbon atoms and six hydrogen atoms. In the C 2 H 6 Lewis structure, there is a single bond between the two carbon atoms, and each carbon is attached with three hydrogen atoms, and none of the atoms has a lone pair. In the periodic table , carbon lies in group 14, and hydrogen lies in group 1. Hence, carbon has four valence electrons and hydrogen has one valence electron. Learn how to find: Carbon valence electrons and Hydrogen valence electrons. We have a total of 14 valence electrons. And when we divide this value by two, we get the value of total electron pairs. Here hydrogen can not be the central atom. Because the central atom is bonded with at least two other atoms, and hydrogen has only one electron in its last shell, so it can not make more than one bond. Now there are only two atoms remaining and both atoms are carbon, so we can assume any one as the central atom.
Lewis diagram for c2h6
The two Carbon atoms C are at the center and they are surrounded by 3 Hydrogen atoms H. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of C2H6. Here, the given molecule is C2H6 ethane. In order to draw the lewis structure of C2H6, first of all you have to find the total number of valence electrons present in the C2H6 molecule. Valence electrons are the number of electrons present in the outermost shell of an atom.
Aladdin torrent
What are some common mistakes students make with Lewis structures? How can I draw a Lewis structure of a compound? These outer hydrogen atoms are forming a duplet and hence they are stable. Let me explain the above image in short. In order to find the total valence electrons in C2H6 molecule , first of all you should know the valence electrons present in carbon atom as well as hydrogen atom. By doing so, you will get the following lewis structure of C2H6. Because the central atom is bonded with at least two other atoms, and hydrogen has only one electron in its last shell, so it can not make more than one bond. How the N3-lewis structure is formed? What are some examples of Lewis structures? Now there are only two atoms remaining and both atoms are carbon, so we can assume any one as the central atom. Amongst these hybrid orbitals, one hybrid orbital will overlap with the 1s-orbital of the Hydrogen atom that produces the sigma bond between a Hydrogen and Carbon atom.
Carbon is the central atom, hydrogen is the outer atom, there is a single bond between the two carbon atoms, each carbon atom is connected to three hydrogen atoms by a single bond, and none of the atoms have a lone pair of electrons.
In the periodic table , carbon lies in group 14, and hydrogen lies in group 1. In short, now you have to find the formal charge on carbon C atoms as well as hydrogen H atoms present in the C2H6 molecule. In order to check the stability of the central carbon C atoms, we have to check whether they are forming an octet or not. This means that they have 8 electrons. Visit our contact page. But as per the rule we have to keep hydrogen outside. Therefore, this structure is the stable Lewis structure of C 2 H 6. C2h6 therefore has a tetrahedral molecular geometry. Stefan V. Save my name, email, and website in this browser for the next time I comment.

Certainly. And I have faced it. Let's discuss this question. Here or in PM.