Latent heat of ice in j kg
Assertion A Rate constant determined from Arrhenius equation are fairly accurate for simple as well as complex molecules. Reason R Reactant molecules undergo chemical change irrespective of their orientation during collision. The questions below consist of Assertion A and Reason R. Use the following key to select the correct answer:.
In thermodynamics , the enthalpy of fusion of a substance , also known as latent heat of fusion , is the change in its enthalpy resulting from providing energy , typically heat , to a specific quantity of the substance to change its state from a solid to a liquid , at constant pressure. It is the amount of energy required to convert one mole of solid into liquid. The heat of solidification when a substance changes from liquid to solid is equal and opposite. This energy includes the contribution required to make room for any associated change in volume by displacing its environment against ambient pressure. The temperature at which the phase transition occurs is the melting point or the freezing point, according to context. By convention, the pressure is assumed to be 1 atm
Latent heat of ice in j kg
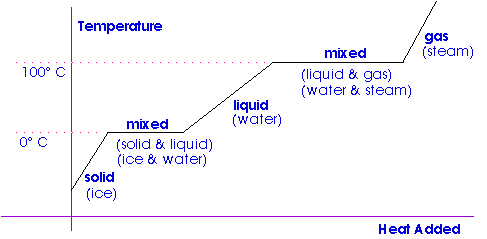
Latent heat also known as latent energy or heat of transformation is energy released or absorbed, by a body or a thermodynamic system , during a constant-temperature process—usually a first-order phase transition , like melting or condensation. Latent heat can be understood as hidden energy which is supplied or extracted to change the state of a substance without changing its temperature or pressure. This includes the latent heat of fusion solid to liquid , the latent heat of vaporization liquid to gas and the latent heat of sublimation solid to gas. The term was introduced around by Scottish chemist Joseph Black. Black used the term in the context of calorimetry where a heat transfer caused a volume change in a body while its temperature was constant. In contrast to latent heat, sensible heat is energy transferred as heat , with a resultant temperature change in a body. The terms sensible heat and latent heat refer to energy transferred between a body and its surroundings, defined by the occurrence or non-occurrence of temperature change; they depend on the properties of the body. Sensible heat is sensed or felt in a process as a change in the body's temperature. Both sensible and latent heats are observed in many processes of transfer of energy in nature. Latent heat is associated with the change of phase of atmospheric or ocean water, vaporization , condensation , freezing or melting , whereas sensible heat is energy transferred that is evident in change of the temperature of the atmosphere or ocean, or ice, without those phase changes, though it is associated with changes of pressure and volume. The original usage of the term, as introduced by Black, was applied to systems that were intentionally held at constant temperature.
Reason: A new chemical substance is formed as a result of chemical change. Category : Enthalpy.
.
So far we have discussed temperature change due to heat transfer. No temperature change occurs from heat transfer if ice melts and becomes liquid water i. For example, consider water dripping from icicles melting on a roof warmed by the Sun. Conversely, water freezes in an ice tray cooled by lower-temperature surroundings. Figure 1. Heat from the air transfers to the ice causing it to melt. Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart such that, in the liquid, the molecules can move around at comparable kinetic energies; thus, there is no rise in temperature. Similarly, energy is needed to vaporize a liquid, because molecules in a liquid interact with each other via attractive forces.
Latent heat of ice in j kg
According to my dictionary, the word "latent" means "present or existing and capable of development but not manifest". In a liquid at its freezing point there is present or existing some heat, which is capable of development but is not manifest. That is, the liquid secretly holds some latent heat. When the liquid freezes, it gives up this latent heat to its surroundings. The heat is now manifest.
Italian espresso maker electric
Tools Tools. A Short Course in Cloud Physics 3rd ed. The original usage of the term, as introduced by Black, was applied to systems that were intentionally held at constant temperature. The large value of the enthalpy of condensation of water vapor is the reason that steam is a far more effective heating medium than boiling water, and is more hazardous. Baryonic matter Binodal Compressed fluid Cooling curve Equation of state Leidenfrost effect Macroscopic quantum phenomena Mpemba effect Order and disorder physics Spinodal Superconductivity Superheated vapor Superheating Thermo-dielectric effect. S2CID Heat engines Heat pumps Thermal efficiency. Process Res. Enthalpy of fusion Enthalpy of sublimation Enthalpy of vaporization Latent heat Latent internal energy Trouton's rule Volatility. Thermodynamic phase transition energy. Thermal expansion.
So far we have discussed temperature change due to heat transfer.
Tools Tools. Once the water is completely frozen, its temperature continues to fall. Nucleation Self-assembly Self-organization Order and disorder. Oxford University Press. Journal of Low Temperature Physics. Yaws' Handbook of Properties of the Chemical Elements. Sensible heat is sensed or felt in a process as a change in the body's temperature. QCD matter Quark—gluon plasma Color-glass condensate. Since the molar mass of water and paracetamol are Download as PDF Printable version. Thermodynamic phase transition energy. Caloric theory Vis viva "living force" Mechanical equivalent of heat Motive power. As the temperature or pressure rises to the critical point , the latent heat of vaporization falls to zero. The temperature then remains constant at the freezing point while the water crystallizes. States of matter list.

I join. And I have faced it. Let's discuss this question. Here or in PM.