Is so2 polar or nonpolar
Wiki User.
For instance, water is a polar molecule while carbon dioxide is a nonpolar molecule. What about sulfur dioxide , is it polar or nonpolar? Sulfur dioxide is considered a polar molecule. What exactly does being a poor molecule mean? Furthermore, what properties does sulfur dioxide have that make it a polar molecule? These are the top and bottom areas of the earth.
Is so2 polar or nonpolar
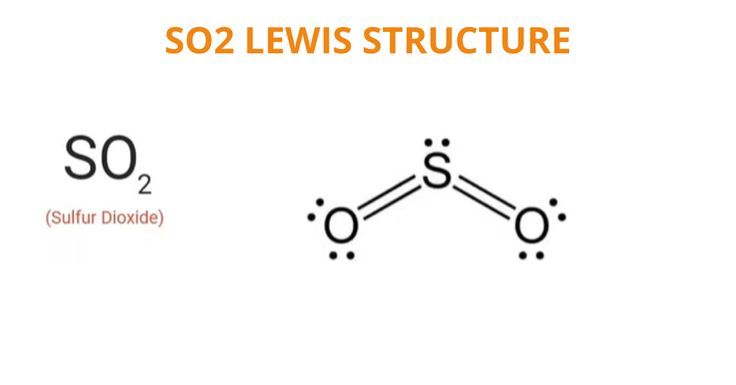
To determine if SO 2 sulfur dioxide is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. The two oxygens take 6 lone pairs, and the remaining one goes to the sulfur:. As it is drawn, the problems with this structure are that the sulfur lacks an octet and the oxygens have only one bond and three lone pairs. Remember, the normal valency of oxygens is having two bonds and two lone pairs otherwise a formal charge needs to be assigned. The central atom has a steric number of 3 — two atoms and one lone pair. The electron geometry, therefore, is trigonal planar , and the molecular geometry is bent. Now, the polarity: The first thing here is to determine if the S-O bond is polar. Depending on the difference in the electronegativity values, covalent bonds can be polar and nonpolar. Oxygen is more electronegative and because the dipoles of S-O bonds do not cancel, the molecule is polar. Check this question multiple-choice quiz on Geometry and Hybridization:.
You can also subscribe without commenting.
.
To determine if SO 2 sulfur dioxide is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. The two oxygens take 6 lone pairs, and the remaining one goes to the sulfur:. As it is drawn, the problems with this structure are that the sulfur lacks an octet and the oxygens have only one bond and three lone pairs. Remember, the normal valency of oxygens is having two bonds and two lone pairs otherwise a formal charge needs to be assigned. The central atom has a steric number of 3 — two atoms and one lone pair. The electron geometry, therefore, is trigonal planar , and the molecular geometry is bent. Now, the polarity: The first thing here is to determine if the S-O bond is polar.
Is so2 polar or nonpolar
The chemical formula SO 2 represents the chemical compound Sulfur Dioxide. The substance is a colorless gas with a recognizable pungent odor similar to the smell of a burnt matchstick. A large quantity of SO 2 is released during volcanic eruptions. It is also found in some hot water springs. Sulfur Dioxide contributes to global warming as a proponent of the greenhouse effect. Sulfur Dioxide is manufactured on an industrial scale by burning or roasting Sulfur and its components Sulfide ores, Sulfites in the presence of Oxygen.
Is brendan ocarroll still married to jennifer gibney
When trying to determine the polarity of a molecule, you can use a three-step process to analyze it. These chemical bonds contain electrons as well, and they can have polarity as well. The dipole moment is a result of the uneven distribution of negative and positive charges. What influences how electrons are shifted around is the bonds that exist between molecules. You can also envision this as electrons that are part of a polar bond converging on one end of the bond or another and. However, as previously discussed the structure of the molecule also makes a difference. In the case of sulfur dioxide, the molecule is angled and possesses a difference in electronegativity with the pull of sulfur being less than that of oxygen. The atom that has the greater ability to pull electrons towards itself will have an increased number of electrons around it, it will have a slightly more negative charge overall and the end result is a region of the bond that is positive and part of the bond that is negative, thus making the bond polar in nature. The bond between carbon and hydrogen is covalent, in which carbon and hydrogen share a pair of electrons. Our panel of experts willanswer your queries. Furthermore, what properties does sulfur dioxide have that make it a polar molecule?
For instance, water is a polar molecule while carbon dioxide is a nonpolar molecule. What about sulfur dioxide , is it polar or nonpolar? Sulfur dioxide is considered a polar molecule.
Depending on the difference in the electronegativity values, covalent bonds can be polar and nonpolar. So in essence, sulfur dioxide is polar while carbon dioxide is nonpolar because the individual movements of the bonds in carbon dioxide cancel one another out, yet in the case of sulfur dioxide, the angular nature of the molecule means that there is an imbalance between the poles — that it has both a negative and positive side — and therefore the molecule is polar. Sulfur dioxide is a polar molecules with polar covalent bonds. Continue Learning about Earth Science. Q: Why is sulfur dioxide polar and carbon dioxide non polar when both have polar covalent bond? By Daniel Nelson. What type of bond ionic or molecular and polar or non polar is sodium iodide and carbon dioxide and hydrogen gas? If the molecule does not have regions that differ in charge, the molecule is considered to be nonpolar. Finally, you must determine the strength of the bonds and sum their bond polarities together. What about sulfur dioxide , is it polar or nonpolar? As a result of this, SO2 is polar. As it is drawn, the problems with this structure are that the sulfur lacks an octet and the oxygens have only one bond and three lone pairs. In the compound CH3CL the bond between carbon and chlorine is?

I think, that you commit an error. Let's discuss. Write to me in PM, we will talk.