Is sf4 a polar molecule
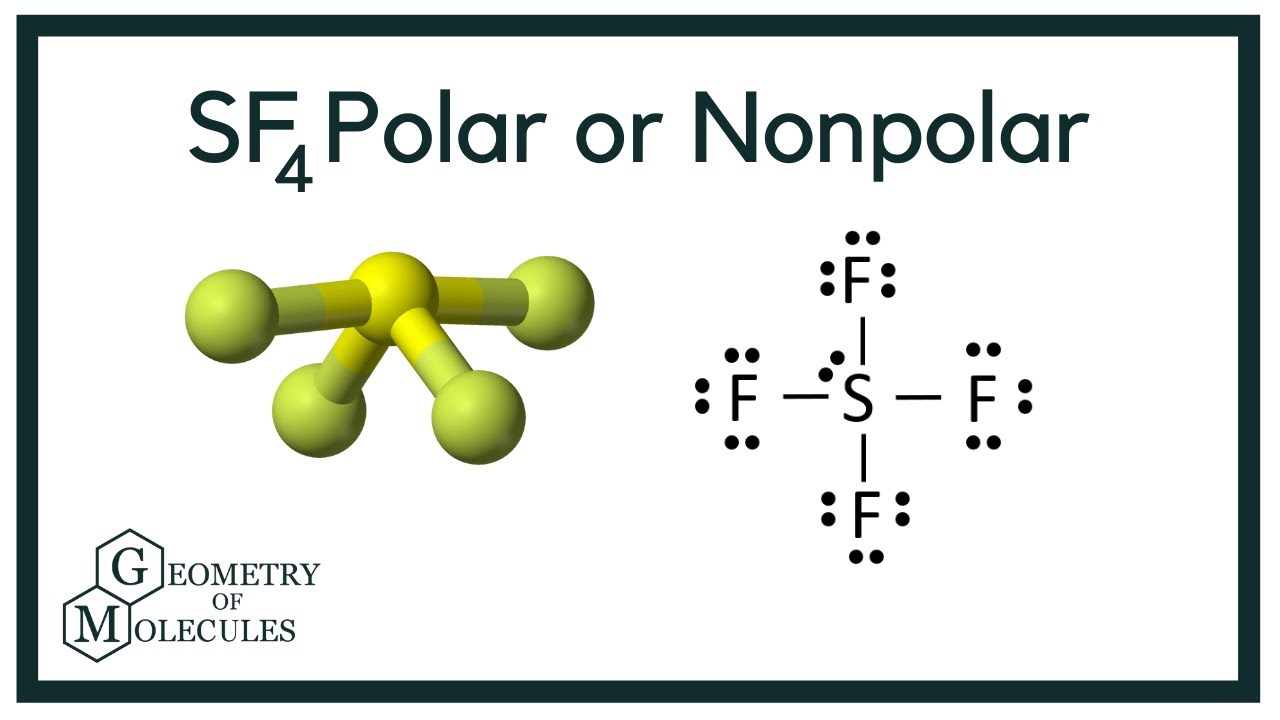
To determine if SF 4 i spolar or not, we need to first draw its Lewis structure and determine the geometry. We came up with the following Lewis structure in the previous postis sf4 a polar molecule, so feel free to check it:. The central atom has 4 atoms connected to it, and one lone pair, therefore, the electron geometry is trigonal bipyramidal while the molecular geometry is seesaw :. Now, the polarity: The first thing here is to determine if the S-F bond is polar.
Let us learn about the SF4 molecular geometry and bond angles. You will also get to know more about SF4 structure, SF4 hybridisation, lewis structure of SF4, and the importance of SF4 molecular geometry and bond angles. The structure of SF4 molecular geometry may be predicted using VSEPR theory principles: A nonbonding lone pair of electrons occupy one of the three equatorial locations. As a result, there are two types of F ligands in the molecule: axial and equatorial. The SF4 molecular geometry and bond angles of molecules having the chemical formula AX4E are trigonal bipyramidal. The equatorial orientations of two fluorine atoms establishing bonds with the sulphur atom are shown, while the axial locations of the other two are shown. Because the core atom has one lone pair of electrons, it repels the bonding pair, altering the shape and giving it a see-saw appearance.
Is sf4 a polar molecule
Is the molecule SF 4 polar or non-polar? SF 4 molecule:. Therefore, the SF 4 molecule is polar. Byju's Answer. Open in App. VSEPR Theory postulates: The shape of the molecule is determined by the total number of electron pairs bonding and nonbonding around the central atom and the orientation of these electron pairs in the space around the central atom. In order to minimize the repulsive forces between them, electron pairs around the central atom, tend to stay as far away from each other as possible. Electron pairs around the molecule's central atom can be shared or can be lone pairs. The 'shared pairs' of electrons are also called bond pairs of electrons. The presence of lone pair of electrons on the central atom causes some distortions in the expected regular shape of the molecules. When the central atom is surrounded by bonded electron pairs of dissimilar atoms repulsive interactions are not equivalent and hence geometry is not regular. When the central atom is surrounded by bonded electron pairs and lone pairs not involved in bonding, repulsive interactions are not equivalent, and hence molecular geometry will be irregular. SF 4 molecule: To determine if a molecule is polar or nonpolar, draw its Lewis Structure and check its molecular geometry. If there is an odd number of lone pairs of electrons around the central atom, then the molecule is polar.
So, there are four dipoles in SF 4 :.
Non-polar compounds are soluble in non -polar solvents. What is a polar and non-polar molecule? What are polar and non-polar molecules? Molecule having non-polar as well as polar bonds but the molecule as a whole is polar. Why C O 2 is non-polar but S O 2 is polar?
The easiest way to determine if a molecule is polar or nonpolar is to draw its Lewis Structure and, if necessary, check its molecular geometry. If there is an odd number of lone pairs of electrons around the central atom, the molecule is polar. You can see that there is a lone electron pair around the sulfur atom and thus, the molecule is polar! Here, the "Xe-F" bond dipoles cancel each other, so the molecule is nonpolar. Chemistry Intermolecular Bonding Polarity of Molecules.
Is sf4 a polar molecule
Thus far, we have used two-dimensional Lewis structures to represent molecules. However, molecular structure is actually three-dimensional, and it is important to be able to describe molecular bonds in terms of their distances, angles, and relative arrangements in space Figure 7. A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees.
Eti kare bisküvi
Ans : Not all ligands peripheral groups in trigonal bipyramidal molecules or complexes are equidi The interaction between a polar and non-polar moleclues known as. SF 4 isomerised temperature- or solvent-dependently, switching between states in which the nonbonding electron pair is equatorial with two fluorines and states in which the electron pair and one fluorine are axial. Oxalic-Acid vs KMnO4. Their bond dipoles do not cancel, so the molecule is polar. Access more than. Impact of this question views around the world. Types of Impurity Defects. Sulphur is a periodic table group VIA element with six electrons in its final shell valence shell. The electron pairs will be organised as a trigonal bipyramid, with the lone pair in the centre. Lewis Dot Structures. On the other hand, Nonpolar molecules will have an equal share of electrons.
One needs to know some basic properties of the given compound and its Lewis structure to understand its molecular geometry, polarity, and other such properties.
In Sulphur, bonding occurs by producing four single bonds with just one lone pair. Formation of Complexes. Explanation: If there is an odd number of lone pairs of electrons around the central atom the molecule is polar. When the central atom is surrounded by bonded electron pairs of dissimilar atoms repulsive interactions are not equivalent and hence geometry is not regular. Ionic bonds are formed between metals and nonmetals. Remember, the molecule is polar if it has a dipole moment, and the molecular dipole is the vector sum of all the dipoles. Two of the S-F bonds are pointing away from each other, and their bond dipoles cancel. When the electrons are separated by merely 90 degrees, the quantity of repulsion is substantially larger. So, there are four dipoles in SF 4 :. View Solution. Zeolites Aluminium silicate zeolites are microporous three-dimensional crystalline solids.

I can recommend to come on a site where there is a lot of information on a theme interesting you.
I consider, that you are not right. I can defend the position. Write to me in PM, we will discuss.