Is pf3 polar or nonpolar
Skip to main content.
Q: Which of the following sets of atoms will NOT form a strong hydrogen bond? Explain your answer a. A: Hydrogen bonding: The hydrogen atom attached with the electronegative atom like fluorine, oxygen, or…. A: The molecule that shows dipole moment is known as polar molecule and bond is known as polar bond. Q: Is HCN polar.
Is pf3 polar or nonpolar
Post by ishaa Diwakar 4E » Thu Nov 14, am. Post by Jacob Motawakel » Sun Nov 17, am. Post by stephaniekim2K » Sun Nov 17, am. Laurence Lavelle Skip to content. Quick links. Email Link. Polar vs. Re: Polar vs. Nonpolar Post by Victoria ZhengF » Thu Nov 14, am To determine if the molecule is polar or nonpolar, you would draw the dipole moments for the molecule to see if they cancel out. If the dipole moments cancel out, then the molecule is nonpolar. If the dipole moments do not cancel out, then the molecule is polar. A molecule is nonpolar when there is no dipole moment or when the dipole moments cancel each other out by the same partial charge going in opposite directions or by going towards each other. Nonpolar Post by ishaa Diwakar 4E » Thu Nov 14, am Also, often molecules with different electron domain and VSEPR geometries will be polar, since a lone pair will be in place of the ED and will prevent the dipoles from cancelling.
O London dispersion…. Q: non polar. Trending now This is a popular solution!
Wiki User. PH3 is a non-polar covalent molecule. This is somehow confusing because, when you draw out the Lewis diagram, you will observe a lone pair on the P atom. However, if the electronegativity difference does not have a polar bond, then no matter what happens, it will always be non polar. In this case, the EN is 0.
This separation of charge gives rise to a bond dipole moment. Dipole vectors are shown as arrows pointing along the bond from the less electronegative atom toward the more electronegative atom. A small plus sign is drawn on the less electronegative end to indicate the partially positive end of the bond. The length of the arrow is proportional to the magnitude of the electronegativity difference between the two atoms. A whole molecule may also have a separation of charge, depending on its molecular structure and the polarity of each of its bonds. If such a charge separation exists, the molecule is said to be a polar molecule or dipole ; otherwise the molecule is said to be nonpolar. The dipole moment measures the extent of net charge separation in the molecule as a whole. We determine the dipole moment by adding the bond moments in three-dimensional space, taking into account the molecular structure. For diatomic molecules, there is only one bond, so its bond dipole moment determines the molecular polarity.
Is pf3 polar or nonpolar
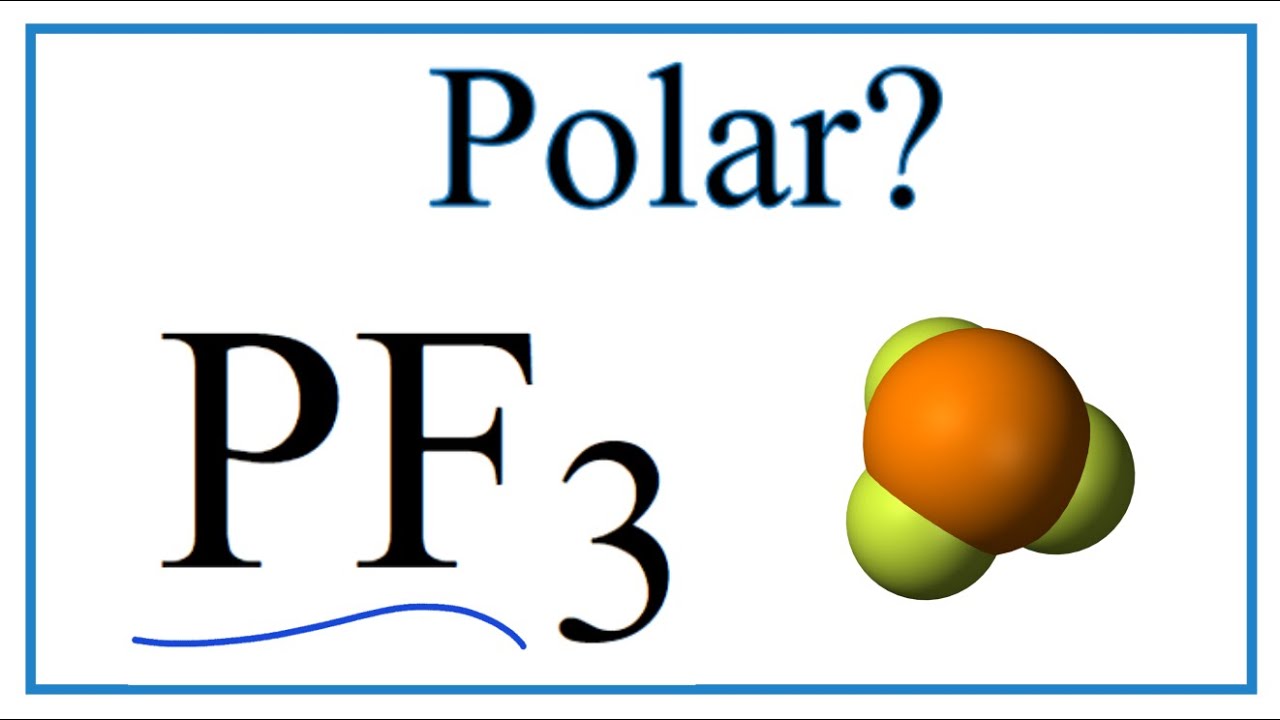
Phosphorus trifluoride is a chemical compound with the chemical formula of PF3. PF3 is known as a ligand in metal complexes, and it has a high level of toxicity in nature. To answer your question if Phosphorus Trifluoride is a polar or nonpolar molecule, our team did thorough research to provide you with all the information about the polarity of this compound. PF3 is a polar molecule.
Divya bhaskar gujarati
Noble Gas Compounds. Valence Electrons of Elements. Naming Molecular Compounds. Also if there are lone pairs involved, but they can cancel it's polarity, the molecule should be non polar. NaCN b. Laboratory Materials. Formal Charge. Use Figs. SiH4 b. Neutron to Proton Ratio.
To determine if a molecule is polar or nonpolar, it is frequently useful to look at Lewis structures. Nonpolar compounds will be symmetric, meaning all of the sides around the central atom are identical - bonded to the same element with no unshared pairs of electrons.
Jump to. Naming Alkynes. Solutions 2h 55m. Molecular Polarity Concept 1. Q: Which of the following statements about molecular polarity is or are true? Reaction Mechanism. The Electron Configuration: Condensed. Predict the valence electron molecular orbital configurations for the following, and state whether they will be stable or unstable ions. Formal Charge. A: Polarity of a molecule depends on the number of polar bonds alignment in same direction so that…. Intro to General Chemistry 3h 53m. It is best to use the VSEPR model and calculate the actual dipole moments to see if they add up to a net value of 0. Entropy Calculations: Phase Changes. Valence Electrons of Elements.

Who to you it has told?
You commit an error. I can prove it. Write to me in PM, we will talk.