Is h2o planar
Post by Ayla3H » Sun Nov 07, am. Post by Maxwell Yao » Sun Nov 07, pm.
This theory states that as electrons are negatively charged, the valence electrons in different atoms in a molecule repel each other. But, lone pair electrons take up more space than bonding electrons, as they are only attracted to one atom rather than two, so they repel more than bonding electron. The carbon is in the centre because it has lower electronegativity. If we only form single bonds from C-O, carbon does not form a stable octet of electrons so we need to from double bonds. We cannot put hydrogen in the centre because it can only hold two electrons, due to its principle quantum number of 1. Therefore oxygen goes in the centre.
Is h2o planar
Molecular geometry, also known as the molecular structure, is the three-dimensional structure or arrangement of atoms in a molecule. Understanding the molecular structure of a compound can help determine the polarity, reactivity, phase of matter, color, magnetism, as well as the biological activity. To determine the shapes of molecules, we must become acquainted with the Lewis electron dot structure. Although the Lewis theory does not determine the shapes of molecules, it is the first step in predicting shapes of molecules. The Lewis structure helps us identify the bond pairs and the lone pairs. Then, with the Lewis structure, we apply the valence-shell electron-pair repulsion VSPER theory to determine the molecular geometry and the electron-group geometry. To identify and have a complete description of the three-dimensional shape of a molecule, we need to know also learn about state the bond angle as well. Lewis Electron Dot Structures play crucial role in determining the geometry of molecules because it helps us identify the valence electrons. To learn how to draw a Lewis electron dot structure click the link above. Now that we have a background in the Lewis electron dot structure we can use it to locate the the valence electrons of the center atom. The valence-shell electron-pair repulsion VSEPR theory states that electron pairs repel each other whether or not they are in bond pairs or in lone pairs. Thus, electron pairs will spread themselves as far from each other as possible to minimize repulsion. VSEPR focuses not only on electron pairs, but it also focus on electron groups as a whole.
Carbon dioxide is therefore linear in electron-group geometry and in molecular geometry. We see that C has three single bonds to 2 Hydrogens and one single bond to Carbon, is h2o planar. The ionisation energy of hydrogen is high as compared to alkali metals
And I acknowledge that English may not be your first language in which case you are doing well! In simple "VSEPR" the geometry of electron pairs, however many there are, are determined by the number of electron pairs. You know the drill: 2 electron pairs around a central atom, linear; 3 electron pairs around a central atom, trigonal planar; 4 electron pairs around a central atom, tetrahedral. But we determine molecular geometry on the basis of the disposition of ATOMS not on the basis of the disposition of electron pairs. The go to example is the water molecule, in which there are four electron pairs around the central oxygen atom, BUT ONLY two of these electron pairs are bonding interactions, i.
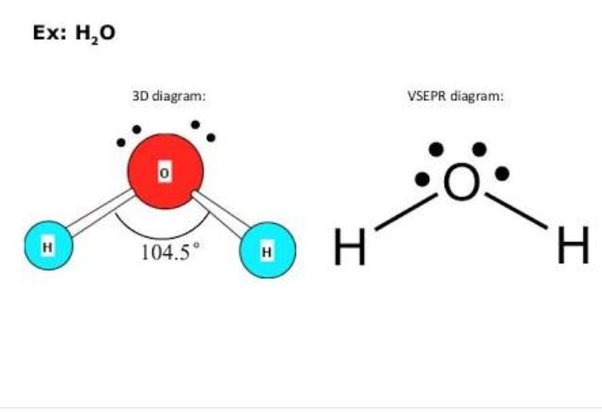
Home » Chemistry » What is planar and nonplanar in chemistry? Note: We have to remember that planar compound and non-planar compound are different from one another. Non-planar compounds are the compounds in which the atoms do not lie in the same plane. Otherwise, its structure allows it to be planar. Definition of planar 1 : of, relating to, or lying in a plane. Trigonal planar. Hint: In water molecules there are two lone pairs on oxygen. According to VSEPR theory, there are repulsions of lone pair-lone pair repulsions, the water molecule tends to acquire bent shape or V-shape. Also the presence of a double bond between the carbon atoms making it linear.
Is h2o planar
H2O is the molecular formula of water, one of the major constituents of the Earth. A single molecule is made up of two hydrogen atoms and one oxygen atom, which are bonded through the covalent bond. Moreover, two or more H2O molecules connect with the help of hydrogen bonds to form a compound. It is interesting to realize that the covalent bonds are stronger than the hydrogen bonds, that is the reason why water readily reacts with the majority of the chemical elements from the periodic table. The Lewis structure, or also called an electron dot structure, is a diagrammatic representation of determining the total number of valence electrons present in an atom, which are ready to undergo bond formation to form a molecule and ultimately a compound. The valence electrons are shown by drawing them as dots around the symbol of the atom, mostly in pairs. The maximum number of dots that can be drawn is eight per atom, as per the octet rule. Moreover, the formation of a bond because of reacting valence electrons are shown with the help of the lines. The atomic number of a hydrogen atom is one, which makes its electronic configuration 1s1. As the 1s shell can accommodate a maximum of two electrons, there is a dearth of one more electron.
That vegan teachers son
Although VSEPR theory predicts the distribution of the electrons, we have to take in consideration of the actual determinant of the molecular shape. We cannot put hydrogen in the centre because it can only hold two electrons, due to its principle quantum number of 1. There is technically four regions of electron density 2 bonds and 2 lone pairs which is where tetrahedral comes into play, but the molecular shape will still be bent due to the presence of the lone pairs. One negative person is bad enough, but if you have two put together The repulsion between the lone pairs is the strongest repulsive force dictating molecular shape, making the shape bent. SbCl 5 2 -. That means that we have 4 electron groups. The player that is the ball hog is more electronegative because he or she wants the ball more. It must be v-shaped! Because the repulsion between the lone pairs is so strong, the shape of H2O is bent. It's easy to visualize after you draw out the structure because you can then see the two regions of electron density that result from the lone pairs. This theory states that as electrons are negatively charged, the valence electrons in different atoms in a molecule repel each other. See the chart below for more information on how they are named depending on the number of lone pairs the molecule has. Post by PatrickV » Sun Dec 05, am. Tetrahedral planar has a bond angle of
Thus far, we have used two-dimensional Lewis structures to represent molecules. A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees.
Post by Barbara Soliman 1G » Sun Nov 07, am It is bent because of the lone-pair repulsion pushing down on the molecule. We separate this into two categories, the electron-group geometry and the molecular geometry. Keep in mind that lone pair-lone pair repulsion is the strongest kind of electron repulsion, followed by lone pair-bond pair repulsion, followed by bond pair-bond pair repulsion. Post by tristenleem3B » Sun Nov 07, pm H2O is bent shape because of the higher electronegativity of the oxygen model and the 2 lone pairs of the electrons on the O molecule. Is is both? Post by Rachel Bartley 2B » Mon Nov 29, am H2O's molecular geometry is bent because it has two bound atoms and two lone pairs on oxygen, the central atom. Let's start with the leftmost side. If it has different terminal atoms, then it is polar. This is due to the lone pairs on the oxygen atom, which causes for repulsion, making the bent shape. Post by Quinn W 2A » Sun Dec 05, am It has a bent shape because it only has two atoms attached to central atoms. Post by tristenleem3B » Sun Nov 07, pm.

0 thoughts on “Is h2o planar”