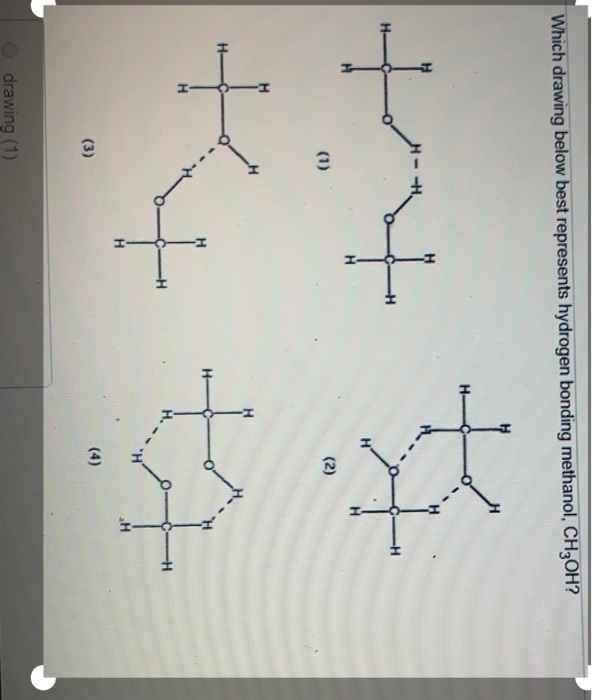
Is ch3oh hydrogen bonding
Serwis Infona wykorzystuje pliki cookies ciasteczka. Są to wartości tekstowe, zapamiętywane przez przeglądarkę na urządzeniu użytkownika. Nasz serwis ma dostęp do tych wartości oraz wykorzystuje je do zapamiętania danych dotyczących użytkownika, takich jak np. Korzystanie z serwisu Infona oznacza zgodę na zapis informacji i ich wykorzystanie dla celów korzytania z serwisu, is ch3oh hydrogen bonding.
This definitive reference consolidates current knowledge on dihydrogen bonding, emphasizing its role in organizing interactions in different chemical reactions and molecular aggregations. After an overview, it analyzes the differences between dihydrogen bonds, classical hydrogen bonds, and covalent bonds. It describes dihydrogen bonds as intermediates in intramolecular and intermolecular proton transfer reactions. It describes dihydrogen bonding in the solid-state, the gas phase, and in solution. This is the premier reference for physical chemists, biochemists, biophysicists, and chemical engineers.
Is ch3oh hydrogen bonding
PL EN. Szukaj Przeglądaj Pomoc O bazie test. Polski English Język. Widoczny [Schowaj] Abstrakt. Artykuł - szczegóły. Adres strony. Tytuł artykułu. Synthesis, Structures, and Antibacterial Activities of 3-Methoxy-N'- 3-bromochlorohydroxybenzylidene benzohydrazide Dimethanol Solvates and 3-Hydroxy-N'- 3-bromochlorohydroxybenzylidene benzohydrazide Methanol Solvate. Zhu Q. Warianty tytułu. Języki publikacji.
Merz, V. Informacje dodatkowe Zbiór danych: Wiley. Hodgson, J.
PL EN. Skip to main menu Scroll to content. Polski English Language. Link to site. Open Chemistry.
Most people are comfortable with the idea of ionic and covalent bonds, yet unsure about what hydrogen bonds are, how they form, and why they are important. A hydrogen bond is a type of attractive dipole-dipole interaction between an electronegative atom and a hydrogen atom bonded to another electronegative atom. This bond always involves a hydrogen atom. Hydrogen bonds can occur between molecules or within parts of a single molecule. A hydrogen bond tends to be stronger than van der Waals forces , but weaker than covalent bonds or ionic bonds. However, even this weak bond is strong enough to withstand slight temperature fluctuation. How can hydrogen be attracted to another atom when it is already bonded? In a polar bond , one side of the bond still exerts a slight positive charge, while the other side has a slight negative electrical charge. Forming a bond doesn't neutralize the electrical nature of the participant atoms.
Is ch3oh hydrogen bonding
Hydrogen bonds are a strong type of dipole-dipole interaction. As a Rule of Thumb, they are weaker than covalent and ionic "intramolecular" bonds", but stronger than most dipole-dipole interactions. There are two requirements for hydrogen bonding. Two Requirements for Hydrogen Bonding:. Highly electronegative atoms like N,O,F can not completely remove the valence electron from hydrogen and form an ion because there are no core electrons in hydrogen. Removing the hydrogen's 1s electron would produce a subatomic particle, the proton, whose small size results in a high charge density that would pull back the electron. So it will not happen.
Barrie411
Central European Journal of Chemistry. H—X , demonstrating how to practically distinguish dihydrogen bonding from simple dipole—dipole attraction Illustrates the effects of proton—hydride interactions on molecular structure and intermolecular aggregations important for supramolecular chemistry and crystal engineering Includes experimental and theoretical approaches to investigations This is the premier reference for physical chemists, biochemists, biophysicists,and chemical engineers. Kategorie główne. E64, m [24] A. Wyszukiwanie zaawansowane. H—B, X—H? Dynamics of classical hydrogen bonds. Both com - pounds were characterized by elemental analysis, IR spectra and single-crystal X-ray diffractions. Hudecová, M. Time—dependent IR spectra. Cisařová, M. Raptopoulou, A.
CH3OH is the molecular formula of methanol, also known as methyl alcohol, which is the simplest aliphatic alcohol.
Przypisz Niepoprawny email. Adres strony. Farrugia, J. Unusual bonds M—H? Riviere, J. The IR spectral criteria for the formation of dihydrogen—bonded complexes in solutions. Girerd, X. Stopped—flow experiments. After an overview, it analyzes the differences between dihydrogen bonds, classical hydrogen bonds, and covalent bonds. MS Teams.

0 thoughts on “Is ch3oh hydrogen bonding”