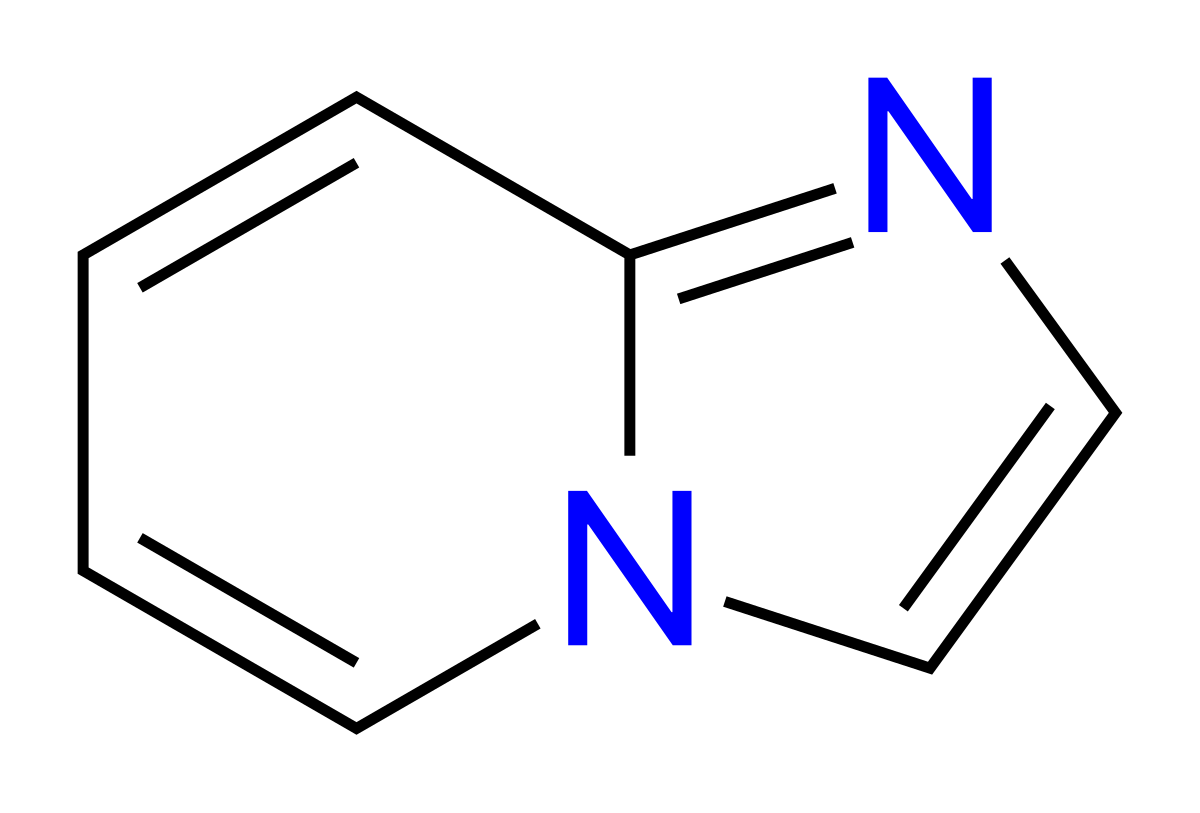
Imidazopyridine
This moiety is also useful in material science because of its structural character. Synthesis of this moiety from the easily available chemicals is desirable due to imidazopyridine tremendous use in the various branches of chemistry. Here we report a review on the synthesis of this scaffold employing different strategies such as condensation, multicomponent reactions, oxidative coupling, tandem reactions, aminooxygenation, imidazopyridine, and hydroamination reactions. Bagdi, imidazopyridine, S.
Federal government websites often end in. The site is secure. Box , United Arab Emirates. Fused pyridines are reported to display various pharmacological activities, such as antipyretic, analgesic, antiprotozoal, antibacterial, antitumor, antifungal, anti-inflammatory, and antiapoptotic. They are widely used in the field of medicinal chemistry. Imidazopyridines IZPs are crucial classes of fused heterocycles that are expansively reported on in the literature. Evidence suggests that IZPs, as fused scaffolds, possess more diverse profiles than individual imidazole and pyridine moieties.
Imidazopyridine
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Aberrant activation of c-Met signalling plays a prominent role in cancer development and progression. Derivatives 6d , 6e and 6f bearing methyl, tertiary butyl and dichloro-phenyl moieties on the triazole ring, respectively, were the compounds with the highest potential. They significantly inhibited c-Met by Molecular docking and dynamics simulation studies corroborated the experimental findings and revealed possible binding modes of the select derivatives with target receptor tyrosine kinases. The results of this study show that some imidazopyridine derivatives bearing 1,2,3-triazole moiety could be promising molecularly targeted anticancer agents against lung and pancreatic cancers. Cancer continues to be a major health burden being the first or second common cause of death before the age of 70 in 91 countries and accounting for 9. Several recent studies have focused on finding new therapies that target specific signalling pathways in cancer cells and in particular on small molecules targeting aberrant kinases 2. Receptor tyrosine kinases RTKs play critical roles in cell proliferation, survival, migration, invasion and other hallmarks of cancer. Aberrant RTKs activation is associated with disease progression in a variety of human malignancies, making them promising drug targets for cancer treatment 3. Hepatocyte growth factor receptor or mesenchymal-epithelial transition factor c-MET is an important RTK essential for several cellular processes 4 , 5.
All compounds were sketched using Marvin Sketch Furthermore, pi—anion, pi—sulfur and pi—pi interactions were colored as dark orange, light imidazopyridine and dark imidazopyridine, respectively. The reaction between 2-chloropyridines and 2 H -azirines provides imidazo[1,2- a ]pyridines, an heterocyclic moiety commonly found in medicinal chemistry leads and drugs.
Potent serine palmitoyl transferase inhibitor. We continue to work to improve your shopping experience and your feedback regarding this content is very important to us. Please use the form below to provide feedback related to the content on this product. By clicking Submit, you acknowledge that you may be contacted by Fisher Scientific in regards to the feedback you have provided in this form. We will not share your information for any other purposes.
Metrics details. Two series of novel imidazo[1,2-a]pyridinecarbohydrazide derivatives have been designed, synthesized, and evaluated for cytotoxic activity. Target compounds were designed in two series: aryl hydrazone derivatives that were devoid of triazole moiety 7a-e and aryl triazole bearing group 11a-e. In vitro cytotoxicity screening was carried out using MTT assay against three human cancer cells including breast cancer MCF-7 , colon cancer HT , and leukemia K cell lines as well as a non-cancer cell line Vero. Compound 7d bearing 4-bromophenyl pendant from aryl hydrazone series exhibited the highest cytotoxic potential with IC 50 values of The molecular mechanism contributing to the anti-proliferative effect of the most potent compound was investigated in silico using Super Pred software and introduced PDGFRA as a plausible target for 7d. Molecular docking and molecular dynamic studies demonstrated Lys and Asp as key residues interacting with the active compound. Overall, 7d could serve as a suitable candidate for further modifications as a lead anticancer structure. Peer Review reports.
Imidazopyridine
This is a preview of subscription content, log in via an institution to check access. Rent this article via DeepDyve. Institutional subscriptions. Dubey, R. Kumar, A. Naidu, and S. Kulkarni, Asian J. CAS Google Scholar. Clark, A. Pessolano, T.
Mtggoldfish
Funding Statement This research received no external funding. Abstract Imidazopyridine scaffold has gained tremendous importance over the past few decades. In addition, B and D are usually a phenyl or substituted phenyl ring in the more promising compounds. Alakananda Hajra. Assay Tools. Moreover, compound 6e showed a critical pi—pi interaction and compounds 6d and 6f showed one hydrogen bond interaction and broad hydrophobic interactions with the active site of PDGFRA. Bechara, A. This dual catalytic system can also be applied to the one-pot, three-step synthesis of 3-thioimidazo[1,2- a ]pyridines from aminopyridines, ketones, and thiols. Close banner Close. You have access to this article. Lin, P. Imidazo [1, 2-a] pyridines susceptible to excited state intramolecular proton transfer: One-pot synthesis via an Ortoleva—King reaction.
An imidazopyridine is a nitrogen containing heterocycle that is also a class of drugs that contain this same chemical substructure. In general, they are GABA A receptor agonists , however recently proton pump inhibitors , aromatase inhibitors , NSAIDs and other classes of drugs in this class have been developed as well.
Sheikhi, S. In general, they are GABA A receptor agonists , however recently proton pump inhibitors , aromatase inhibitors , NSAIDs and other classes of drugs in this class have been developed as well. Gholampour, M. In addition, the results indicated that the introduction of lipophilic substitutes at the para position of the benzyl pendant resulted in higher inhibitory potential compared to the meta -substituted compounds. Sci Rep 11 , Li, Synlett , , Vuillermet, J. Recombinant Proteins. Buffers and Standards. Google Scholar Duan, Y. Jones, G. In addition, a few derivatives exhibited significant enzyme inhibition activity against S.

What curious topic
Quite right! It is good thought. I call for active discussion.
Rather good idea