If4 shape
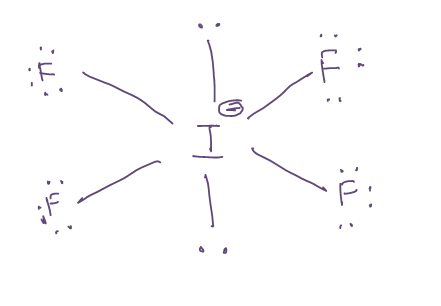
The iodine atom will be the central atom. It will form four single bonds with the fluorine atoms, if4 shape, for a total of 8 out of the 36 valence electrons available. Each of the four fluorine if4 shape will have 3 lone pairs of electrons attached, which brings thte total number of valence electrons used to
Skip to main content. Table of contents. Intro to General Chemistry 0. Classification of Matter. Chemical Properties. Physical Properties.
If4 shape
.
Rutherford Gold Foil Experiment. Naming Ethers. Body Centered Cubic Unit Cell.
.
The Lewis structure of IF4- Iodine Tetrafluoride Ion involves a central iodine atom bonded to four fluorine atoms with one lone pair, totaling 36 valence electrons 7 from iodine, 7 from each of the four fluorines, plus 1 additional for the negative charge. This results in a square pyramidal geometry. The extra electron gives the ion a -1 charge, concentrated on the iodine. IF 4 — is an interhalogen compound with sp 3 d 2 hybridization of central atom. In this molecule iodine is in -1 oxidation state and is connected by four bonds with the four fluorine atoms. Actual structure of this molecule is square planar with a bond angle 90 0. Though the actual geometry of IF 4 — is octahedral. Lewis structure , introduced by Gilbert.
If4 shape
Skip to main content. Table of contents. Intro to General Chemistry 0.
The italian job พากย์ ไทย เต็ม เรื่อง
Dipole Moment. Each of the four fluorine atoms will have 3 lone pairs of electrons attached, which brings thte total number of valence electrons used to The Quadratic Formula. Root Mean Square Speed. Oxide Reactions. Triprotic Acids and Bases. Intro to Electrochemical Cells. Main Group Elements: Periodic Trends. Complex Ions: Formation Constant. Gamma Emission.
The Lewis electron-pair approach can be used to predict the number and types of bonds between the atoms in a substance, and it indicates which atoms have lone pairs of electrons. This approach gives no information about the actual arrangement of atoms in space, however. Keep in mind, however, that the VSEPR model, like any model, is a limited representation of reality; the model provides no information about bond lengths or the presence of multiple bonds.
Collision Theory. Hydrogen Isotopes. Balancing Redox Reactions: Acidic Solutions. Density of Geometric Objects. Internal Energy. Ester Reactions: Esterification. Periodic Trend: Atomic Radius. Root Mean Square Speed. The Electron Configuration: Condensed. The Quadratic Formula. Electron Configurations of Transition Metals: Exceptions.

0 thoughts on “If4 shape”