How many pi bonds in a triple bond
Sigma and pi bonds are types of covalent bonds that differ in the overlapping of atomic orbitals. Covalent bonds are formed by the overlapping of atomic orbitals. Sigma bonds are a result of the head-to-head overlapping of atomic orbitals whereas pi bonds are formed by the lateral overlap of two atomic orbitals.
Our minds can handle two electrons interacting with one another in a sphere of space. But then we start putting in double bonds and triple bonds. So we need a more complex picture that works for all these electrons. The hybridization model helps explain molecules with double or triple bonds see figure below. The entire molecule is planar.
How many pi bonds in a triple bond
Post by » Sat Dec 04, pm. Laurence Lavelle Skip to content. Quick links. Email Link. C and N are triple bonded and each atom has one lone pair. Both C and N are sp hybridized because they both have two regions of electron density. So, the sigma bond between them would be sigma C 2sp, N 2sp. Since both atoms have two unhybridized p orbitals conservation of orbitals , would we notate the two pi bonds as pi C 2p2, N 2p2? Or is each pi bond pi C 2p, N 2p since one pi bond is attributed to one unhybridized p orbital? Should we write pi C 2px, N 2px and pi C 2py, N 2py? What is the proper notation? I'm not entirely sure whether you could clump the two pi bonds together and just write 2pi C 2p, N 2p , but look back at problem 2. Hope this helps!
How are the ethyne pi bonds oriented in relation to each other?
.
A description of the double bond is the sigma-pi model shown in Figure 1. In this case only two of the p orbitals on each C atom are involved in the formation of hybrids. Two of these hybrids from each C atom overlap with H 1 s orbitals, while the third overlaps with an sp 2 hybrid on the other C atom. This orbital has no nodes: electron density exists continuously from around one atom to the other atom. To view the sigma bonding orbital, select N6.
How many pi bonds in a triple bond
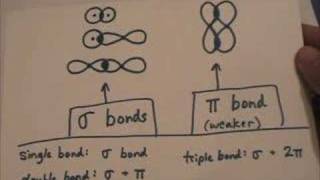
Single, double, and triple bonds are three types of covalent bonds mainly involving nonmetals. Atoms form these bonds as a way of obtaining the most stable electron configuration, according to the octet rule. Since metals usually need more than three electrons to achieve this, they less commonly form these types of bonds. Here is a closer look at single, double, and triple bonds, along with examples of each type and their properties. According to Langmuir, covalence is the number of pairs of electrons shared between an atom and its neighbor. A single bond is a covalent bond that occurs when two atoms share one electron pair. Atoms that form this type of bond are one electron away from a noble gas configuration, so elements participating in single bonds are hydrogen and the halogens, with each other or with other elements. There are some exceptions. The notation for a single bond is a single dash between the atoms, such as H-H or Cl-Cl. Usually, a single bond is a sigma bond, although the bond in diboron B 2 is a pi bond.
Sazon miami beach
Put your understanding of this concept to test by answering a few MCQs. In general, single bonds between atoms are always sigma bonds. Triple bonds are comprised of one sigma bond and two pi bonds. Post My Comment. Or is each pi bond pi C 2p, N 2p since one pi bond is attributed to one unhybridized p orbital? Re: Pi bonds in triple bonds Post by Natalie Quilala 1I » Mon Dec 06, am For triple bond, the main component to understand is that there will be 2 pi bonds and 1 sigma bond making up the three bonds. This plane contains the six atoms and all of the sigma bonds. Search site Search Search. The hybridization model helps explain molecules with double or triple bonds see figure below. Should we write pi C 2px, N 2px and pi C 2py, N 2py? Pi Bonds are generally weaker than sigma bonds, owing to the significantly lower degree of overlapping. Go back to previous article. Therefore, the total number of sigma bonds in a benzene molecule is Review What is the hybridization around each carbon in ethene? C and N are triple bonded and each atom has one lone pair.
Our minds can handle two electrons interacting with one another in a sphere of space.
The three most common overlap conditions that result in sigma bonds are:. Watch Now. I'm not entirely sure whether you could clump the two pi bonds together and just write 2pi C 2p, N 2p , but look back at problem 2. Who is online Users browsing this forum: No registered users and 0 guests. This type of covalent bonding is illustrated below. Components Of Ecosystem. The pi bond is the "second" bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the molecule. Pi Bonds are generally weaker than sigma bonds, owing to the significantly lower degree of overlapping. It is important to note that a combination of sigma and pi bonds is always stronger than a single sigma bond. But then we start putting in double bonds and triple bonds.

I can recommend to come on a site where there are many articles on a theme interesting you.