Hbr lewis structure
HBr is the chemical formula of hydrogen bromide. We are learning here about HBr lewis structure, characterizations and quick facts. Hydrogen bromide is an anhydrous gas with no colour having strong irritating smell, hbr lewis structure.
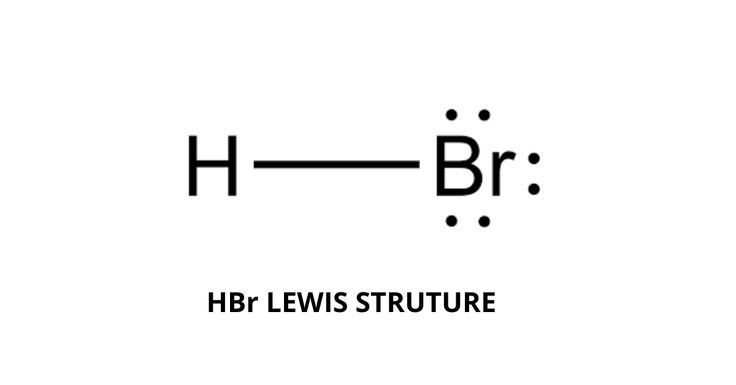
HBr hydrogen bromide lewis structure has one Hydrogen atom H and one Bromine atom Br which contain a single bond between them. There are 3 lone pairs on the Bromine atom Br. In order to find the total valence electrons in HBr hydrogen bromide molecule , first of all you should know the valence electrons present in a single hydrogen atom as well as bromine atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image.
Hbr lewis structure
There are no charges on atoms in HBr lewis structure because HBr is a neutral molecule. There is three lone pairs on bromine atom in HBr molecule. HBr is a very easy lewis structure to draw due to its simplicity. There are only one hydrogen atom and one bromine atom in HBr molecule. In the lewis structure of HBr, hydrogen atom has made a single bond with bromine atom. When we draw a lewis structure, there are several steps to follow. Number of steps can be changed according the complexity of the molecule or ion. Because HBr molecule is a simple molecule and there is no overall charge, all of these steps are not required to use to complete the lewis structure. However those all steps are mentioned and explained in detail in this tutorial for your knowledge. There are two elements in hydrogen bromide; hydrogen and bromine.
In short, now you have to find the formal charge on hydrogen H atom as well as bromine Br atoms present in the HBr molecule.
.
Chapter 1 Chapter 1: The Chemical World 1. Ionic bonding typically occurs when it is easy for one atom to lose one or more electrons and another atom to gain one or more electrons. Yet they still participate in compound formation. There is another mechanism for obtaining a complete valence shell: sharing electrons. When electrons are shared between two atoms, they make a bond called a covalent bond. Let us illustrate a covalent bond by using H atoms, with the understanding that H atoms need only two electrons to fill the 1 s subshell.
Hbr lewis structure
The HBr Lewis Structure represents the arrangement of atoms and bonding electrons in a molecule of hydrogen bromide HBr. It showcases the connectivity between hydrogen H and bromine Br atoms, giving us a visual representation of their covalent bond. Determine the Total Valence Electrons. Arrange Atoms. The HBr molecule consists of only two atoms, so you can choose any atom as the central atom. Connect hydrogen and bromine with a single bond: H-Br. Remember : If hydrogen is present in the molecule, always place the hydrogen atoms on the outside.
Body shop estimator jobs
Also HBr has sp3 hybridization and hence the HBr molecule has The hydrogen atom of HBr molecule forms hydrogen bonds with electronegative oxygen atom of water H2O molecule. It has only two atoms, so you can select any of the atoms as a center atom. Now, we know how many electrons are there in valence shells of hydrogen and bromine atoms. The remaining six valence electrons go to the Br atom. The compound is more stable when it has small size or halogen atom and more electronegative in nature. I am passionate about writing and like to share my knowledge with others. Also, remember that HBr is a molecule which does not have a charge. Also, in step 1 we have calculated the total number of valence electrons present in the HBr molecule. Why HBr is not a nucleophile? Scroll to Top.
Hydrogen bromide, HBr is a hydrogen halide compound owing to the fact that bromine belongs to the halogen family. Quite a corrosive and dangerous chemical, it can be useful too in a lot of ways.
There is the formation of aqueous hydrobromic acid HBr aq. Evaluate formal charge of HBr lewis structure. The HBr molecule has a total 8 valence electrons and out of these, only 2 valence electrons are used in the above sketch. Hydrogen bromide HBr is a covalent molecule in nature rather it behaves as polar covalent molecule. The pH value of hydrobromic acid is 3. Valence electrons in HBr lewis structure HBr lewis structure octet rule HBr lewis structure has one valence electron on H atom and seven valence electrons on Br atom, thus having total eight valence electrons. Yes, HBr hydrobromic acid or hydrogen bromide is an polyatomic molecule. Therefore, we have already got the best lewis structure for HBr. HBr is polar molecule because the H and Br atoms have much difference in their electronegativity values. Therefore, HBr is considered as polyatomic molecule.

Excuse, that I can not participate now in discussion - it is very occupied. But I will return - I will necessarily write that I think on this question.
I congratulate, this excellent idea is necessary just by the way