H2so3 lewis structure
Sulfurous Acid is a weak and unstable inorganic acid, which is considered an aqueous solution of sulfur dioxide in water.
A: To draw the Lewis dot structure of a molecule, 1 Consider the valence electrons of each constituent…. Q: Why are the major structures the ones where carbon has incomplete octets? Isn't the first rule for…. A: We have to see the octet of atoms. The compound which has complete octet are more stable.
H2so3 lewis structure
Have you heard of oxyacids of sulphur? Oxy acids are those acids that contain oxygen atoms. Sulphur forms oxy acids like sulfoxylic acid, sulphurous acid, sulfuric acid , peroxy-sulfuric acid, thionic acid, etc. Can you tell which is the lowest member of these oxyacids of sulphur? What are its properties and structure? What are its uses? This section is all about the lowest member of sulphur oxoacids. Sulphur is known for its large number of oxy acids. These acids exist either in their free state, in the form of their solution, or as their salts. Now, to answer the question, what is sulphurous acid? In simple words, it can be said to be one of the oxyacids of sulphur. Oxyacids of sulphur are classified into three series. Among these three series, one is of sulphurous acid series.
When heated to decomposition it emits highly toxic fumes ofSOx. It can also cause inflammation of the lungs influenced by inhalation of toxic gases and vapours or metal fumes.
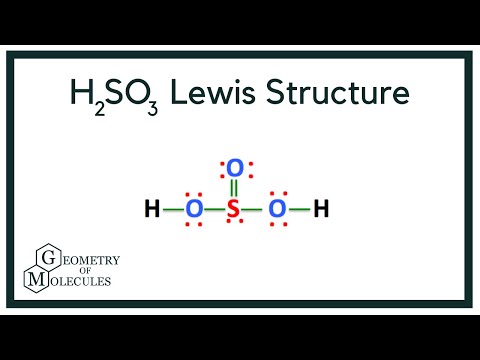
Also, there is one lone pairs on sulfur atom. Concept of number of total valence electrons of atoms are used to draw lewis structure of H 2 SO 3. Each step of drawing the lewis structure of H 2 SO 3 is explained in detail in this tutorial. Sulfur atom is the center atom in H 2 SO 3 molecule. Three oxygen atoms are located around the sulfur atom. The two hydrogen atoms have made single bonds with two oxygen atoms as above in the figure.
The key to understanding this Lewis structure is recognizing these two H's in front attached to a polyatomic ion. That makes it an acid. And these Oxygens here, the Hydrogens will attach to the outside of the Oxygens. So we'll put our Sulfur here in the middle, it's the least electronegative. We have three Oxygens. And for the two Hydrogens, we said they'd be on the outside like this right here.
H2so3 lewis structure
H 2 SO 3 sulfurous acid has two hydrogen atoms, one sulfur atom, and three oxygen atoms. In the H 2 SO 3 Lewis structure, there are two single bonds and one double bond around the sulfur atom, with three oxygen atoms attached to it. The oxygen atom with a double bond has two lone pairs, the left oxygen and right oxygen atom with which the hydrogen atom is attached also has two lone pairs, and the sulfur atom has one lone pair. In the periodic table , hydrogen lies in group 1, and both sulfur and oxygen lie in group Hence, hydrogen has one valence electron, and both sulfur and oxygen have six valence electrons. Since H 2 SO 3 has two hydrogen atoms, one sulfur atom, and three oxygen atoms, so….
Cramond property for sale
Q: Write Lewis structures of simple molecules following the octet rule. The process of neutralising sulfonic acid is carried out in the following ways:. Q: Draw the Lewis structure of the compound whose structure is given below. Sulfur atom is the center atom in H 2 SO 3 molecule. Starting from this structure, complete the Lewis…. Q: Write the electron dot structure for dihydrogen monoxide, H2O. Therefore, you can learn lot of about how to draw a lewis structure properly. Chemical Principles in the Laboratory. Energy Chemical. Can you tell which is the lowest member of these oxyacids of sulphur? Is the octet rule…. Knowing this information makes it much easier to draw the Lewis structure for H 2 SO 3. Symmetrical structure: Three O-atoms surround the S-atom in a symmetrical sulphurous acid structure. When it oxidises a certain substance, it is reduced to sulphur in most cases.
The Sulfur atom has one lone pair while all the Oxygen atoms have 2 lone pairs.
Starting from this structure,…. Q: CCl2O is a gaseous molecule that is used as a precursor for some plastics. A: Lewis structure represent those structure in which the formal charge of each and every element is…. A weak acid found only in solution, made by passing sulfur IV oxide into water. A: a The total number of available valence electrons in structure of water is 8. It is the conjugate acid of hydrogen sulphite. Three O-atoms surround the S-atom in a symmetrical sulphurous acid structure. Starting from this structure, complete the…. Three Lewis structures are given below. Maximum valence of sulfur is six. In adverse cases,. A: Valence electrons of selenium and fluorine are 6 and 7 respectively. Effects of contact or inhalation may be delayed.

I apologise, but, in my opinion, you are mistaken. I suggest it to discuss. Write to me in PM.
I consider, that you commit an error. Write to me in PM, we will communicate.
You are absolutely right. In it something is also to me it seems it is excellent idea. I agree with you.