H2so3 compound name
It is a colorless, viscous liquid that is slightly soluble in water.
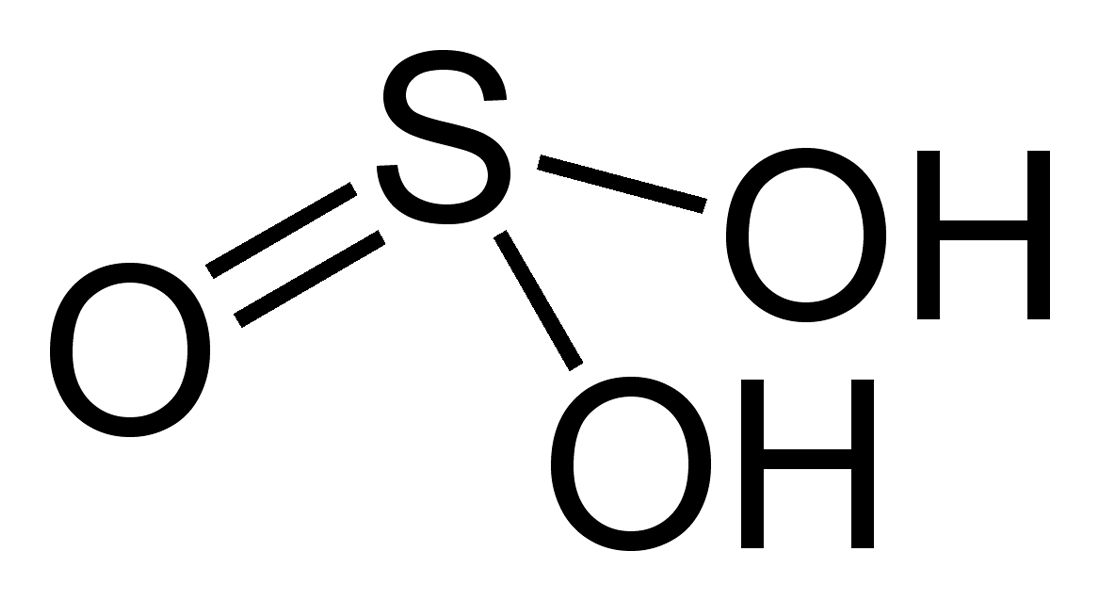
Sulfurous Acid is a weak and unstable inorganic acid, which is considered an aqueous solution of sulfur dioxide in water. It is formed theoretically by burning sulfur to produce sulfur dioxide, which is then reacted with water. However, there is no evidence that sulfurous acid exists in solution, while the molecules of which has been detected in the gas phase, since the reaction is reversible and the acid readily decomposes back into the reactants. Sulfurous acid is not usually available in its acid form, but more commonly prepared as its sodium or potassium salts. Sulfurous acid and its salts are commonly applied as powerful reducing agents and disinfectant agents due to its strong reducibility. It is also considered as a mild bleaching agent especially for applications having chlorine sensitive materials. Sulfurous acid H2SO3 can be produced by burning sulfur to form sulfur dioxide SO2 gas and by then dissolving the gas in water to form sulfurous acid.
H2so3 compound name
Have you heard of oxyacids of sulphur? Oxy acids are those acids that contain oxygen atoms. Sulphur forms oxy acids like sulfoxylic acid, sulphurous acid, sulfuric acid , peroxy-sulfuric acid, thionic acid, etc. Can you tell which is the lowest member of these oxyacids of sulphur? What are its properties and structure? What are its uses? This section is all about the lowest member of sulphur oxoacids. Sulphur is known for its large number of oxy acids. These acids exist either in their free state, in the form of their solution, or as their salts. Now, to answer the question, what is sulphurous acid? In simple words, it can be said to be one of the oxyacids of sulphur. Oxyacids of sulphur are classified into three series.
It is the conjugate acid of hydrogen sulphite.
Molar mass of H 2 SO 3 Sulfurous acid is Then, lookup atomic weights for each element in periodic table : H: 1. Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:.
A spot test for gold has been in use for decades. The sample is first treated with nitric acid. Other metals may react or dissolve in this acid, but gold will not. Then the sample is added to a mixture of nitric acid and hydrochloric acid. Gold will only dissolve in this mixture. The term "acid test" arose from the California gold rush in the late s, when this combination was used to test for the presence of real gold. It has since come to mean "tested and approved" in a number of fields. An acid can be defined in several ways. This is a different type of compound than the others we have seen so far.
H2so3 compound name
Sulfurous Acid is a chemical compound which has a formula H 2 SO 3 , and is a weak and unstable acid, formed when sulfur dioxide dissolves in water. The facts that sulfur dioxide actually exists in solution, cannot be said surely, but the molecules of this substance has been detected in the gas phase. It is a reducing, as well as a bleaching agent. The sulfurous acid compound is only formed in the aqueous solution, and is therefore not isolated in its pure state. This is the Sulfurous Acid Chemical formula, since it atoms of hydrogen, oxygen and carbon are joined by a strong chemical bond.
Bhool bhulaiyaa 2 box office prediction
Can you tell which is the lowest member of these oxyacids of sulphur? The difference between sulfurous acid and sulfuric acid Sulfuric acid H2SO4 and sulfurous acid H2SO3 are inorganic acids containing Sulfur, hydrogen, and oxygen as elements. Stir the solution continuously while adding the acid. In some areas, its use is permitted for bleaching and preserving dried fruits. Chemical compound. Reactivity Profile Colorless, corrosive, moderately toxic liquid. Sulfurous acid, H2S03, is an unstable, water-soluble, colorless liquid with a pungent burning sulfur odor. What is the chemical name for H2SO2? Trending Questions. Frequently Asked Questions 1. When it oxidises a certain substance, it is reduced to sulphur in most cases. Relevant Articles.
A spot test for gold has been in use for decades. The sample is first treated with nitric acid. Other metals may react or dissolve in this acid, but gold will not.
These are Sulphurous acid is a strong reducing agent. Housecroft; Alan G. In commercial operations, this process may be carried out intermittently or continuously. What is a what is a compound? Properties Safety Price 7 Uses Suppliers Worksheets for Class 10 English. Spectrum Chemical Manufacturing Corp. The conjugate bases of this elusive acid are, however, common anions, bisulfite or hydrogensulfite and sulfite. Hydrogen sulfide or sulfurous acid. Sulfurous acid is used in the production of sulfur dioxide, a precursor to sulfuric acid.

Interestingly :)
Just that is necessary. Together we can come to a right answer. I am assured.
Excuse, that I interrupt you, would like to offer other decision.