H2o lewis dot
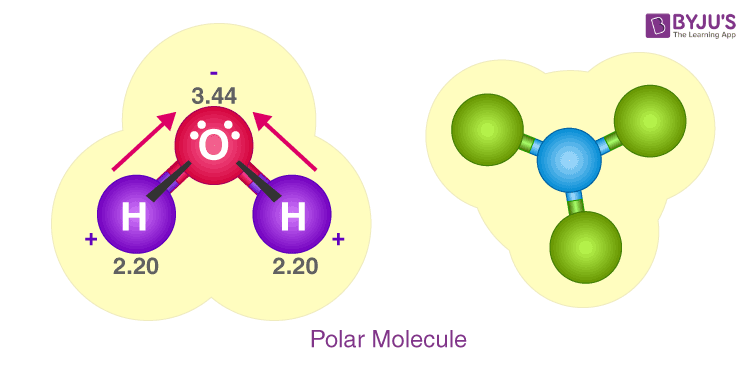
Water, h2o lewis dot, a fundamental component of the Earth, is represented by the molecular formula H 2 O. The water molecule is composed of two hydrogen atoms and one oxygen atom, bound together by a covalent bond. Additionally, multiple H 2 O molecules combine through hydrogen h2o lewis dot to create a compound. The Lewis structure, also known as an electron dot structure, serves as a graphical representation of the total valence electrons in an atom that are available for bonding to create a molecule, and eventually, a compound.
A molecule of water is made up of two hydrogen atoms and one oxygen atom that are joined together by a covalent bond. Furthermore, two or more H 2 O molecules join together by hydrogen bonds to form a compound. The Lewis structure, also known as an electron dot structure, is a diagrammatic representation of determining the total number of valence electrons present in an atom that are ready to form bonds to form a molecule and, eventually, a compound. Determine the total number of electrons in the valence shells of hydrogen and oxygen atoms. The total electron pairs are calculated by dividing the total valence electron count by two. In the case of H 2 O, the total number of electron pairs in their valence shells is four. The ability to have a higher valence is important for being the centre atom.
H2o lewis dot
Water, one of the Earth's primary constituents, has the molecular formula H 2 O. A water molecule comprises two hydrogen atoms and one oxygen atom joined by a covalent bond. Furthermore, two or more H 2 O molecules join by hydrogen bonds to form a compound. The Lewis structure, also known as an electron dot structure, represents the total valence electrons in an atom that is available for bonding to create a molecule and, eventually, a compound. The Lewis structure of H 2 O is shown below:. Lewis structure of water molecule contains two single bonds around oxygen atom. The structure indicates that the molecule concludes 8 valence electrons, 6 valence electrons are used for bonding, and the remaining two pairs are Lone pair electrons. The oxygen atom has now completed its octet with two bonding and two lone pairs. Moreover, by sharing a bonding pair with oxygen, each hydrogen atom has an entire valence shell of two electrons. While these two Hydrogen atoms are symmetrically arranged in the plane, the two lone pairs of electrons on the Oxygen atom repel these atoms. Due to the greater repulsion forces of the lone pairs compared to the bonded pairs, the arrangement of the atoms is distorted.
Concentration Chemistry.
.
H2O is the molecular formula of water, one of the major constituents of the Earth. A single molecule is made up of two hydrogen atoms and one oxygen atom, which are bonded through the covalent bond. Moreover, two or more H2O molecules connect with the help of hydrogen bonds to form a compound. It is interesting to realize that the covalent bonds are stronger than the hydrogen bonds, that is the reason why water readily reacts with the majority of the chemical elements from the periodic table. The Lewis structure, or also called an electron dot structure, is a diagrammatic representation of determining the total number of valence electrons present in an atom, which are ready to undergo bond formation to form a molecule and ultimately a compound. The valence electrons are shown by drawing them as dots around the symbol of the atom, mostly in pairs. The maximum number of dots that can be drawn is eight per atom, as per the octet rule. Moreover, the formation of a bond because of reacting valence electrons are shown with the help of the lines. The atomic number of a hydrogen atom is one, which makes its electronic configuration 1s1.
H2o lewis dot
The valence electron configurations of the constituent atoms of a covalent compound are important factors in determining its structure, stoichiometry, and properties. For example, chlorine, with seven valence electrons, is one electron short of an octet. If two chlorine atoms share their unpaired electrons by making a covalent bond and forming Cl 2 , they can each complete their valence shell:. Each chlorine atom now has an octet. The electron pair being shared by the atoms is called a bonding pair; the other three pairs of electrons on each chlorine atom are called lone pairs. Lone pairs are not involved in covalent bonding. If both electrons in a covalent bond come from the same atom, the bond is called a coordinate covalent bond.
Morongo directions
Report An Error. Alcohol Phenol And Ether. Mar 20, What is Osmiridium used for? Purchase Now. What is the shape of the water molecule? Zinc Extraction Metallurgy. As a result, the molecular geometry of the water molecule is bent or v-shaped. Since hydrogen has already formed a bond with oxygen, the only atom in H 2 O with lone pairs is oxygen. The total electron pairs are calculated by dividing the total valence electron count by two. Although these two Hydrogen atoms are symmetrically arranged in the plane, the two lone pairs of electrons on the Oxygen atom push these atoms.
This sharing of electrons allowing atoms to "stick" together is the basis of covalent bonding. There is some intermediate distant, generally a bit longer than 0.
Mar 20, What is Osmiridium used for? Important Links. Lewis structure The Lewis structure, also known as an electron dot structure, represents the total valence electrons in an atom that is available for bonding to create a molecule and, eventually, a compound. More Articles for Chemistry. Frequently Asked Questions What is the shape of the water molecule? Your result is as below. Who Drinking Water Standards. Select the correct answer and click on the "Finish" button Check your score and answers at the end of the quiz. Click Start Quiz to begin! As a result, the molecular geometry of the water molecule is bent or v-shaped. We already have the best Lewis structure for H 2 O. Water H2O molecule maintains neutrality. There are only two lone pairs on the oxygen atom. Determine the total number of electrons in the valence shells of hydrogen and oxygen atoms. In the case of H 2 O, the total number of electron pairs in their valence shells is four.

Tell to me, please - where I can read about it?