Formal charge of nitrogen
How do you calculate the formal charge of all atoms in NO 3 -? Formal charges are represented as the actual charges on any atom within a molecule, for which we can use the formula as;, formal charge of nitrogen. Step 2: Structure of NO 3 -for understanding bonding within it For NO 3 -the formal charges on each atom can be depicted by analyzing its structure given by.
It is more important that students learn to easily identify atoms that have formal charges of zero, than it is to actually calculate the formal charge of every atom in an organic compound. Students will benefit by memorizing the "normal" number of bonds and non-bonding electrons around atoms whose formal charge is equal to zero. Formal charge is assigned to an atom in a molecule by assuming that electrons in all chemical bonds are shared equally between atoms, regardless of relative electronegativity. To calculate formal charges, we assign electrons in the molecule to individual atoms according to these rules:. The formal charge of each atom in a molecule can be calculated using the following equation:. A neutral nitrogen atom has five valence electrons it is in group From the Lewis structure, the nitrogen atom in ammonia has one lone pair and three bonds with hydrogen atoms.
Formal charge of nitrogen
Too much emphasis can easily be placed on the concept of formal charge, and the mathematical approach used in the textbook is hard to justify. In this course, you will certainly need to be able to recognize whether a given species carries a charge i. It is sometimes possible to write more than one Lewis structure for a substance that does not violate the octet rule, as we saw for CH 2 O, but not every Lewis structure may be equally reasonable. In these situations, we can choose the most stable Lewis structure by considering the formal charge on the atoms, which is the difference between the number of valence electrons in the free atom and the number assigned to it in the Lewis electron structure. The formal charge is a way of computing the charge distribution within a Lewis structure; the sum of the formal charges on the atoms within a molecule or an ion must equal the overall charge on the molecule or ion. A formal charge does not represent a true charge on an atom in a covalent bond but is simply used to predict the most likely structure when a compound has more than one valid Lewis structure. To calculate formal charges, we assign electrons in the molecule to individual atoms according to these rules:. A neutral nitrogen atom has five valence electrons it is in group Substituting into the formula, we obtain. A neutral hydrogen atom has one valence electron. Using Equation 4. The hydrogen atoms in ammonia have the same number of electrons as neutral hydrogen atoms, and so their formal charge is also zero.
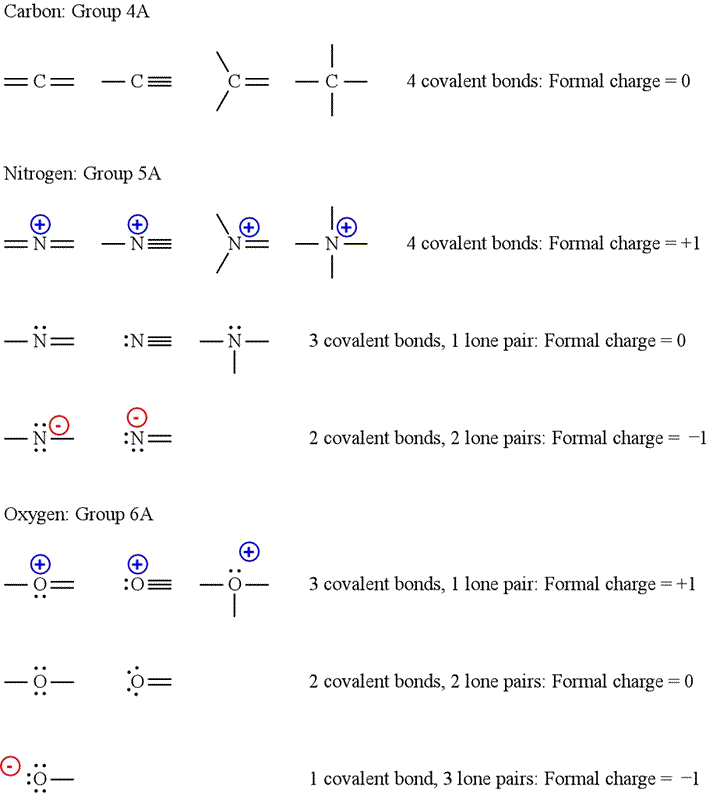
The pattern for a formal charge of negative one on nitrogen would be two bonds, here are the two bonds, and two lone pairs of electrons. So this has a formal charge of plus one, so we have another pattern to think about here. If we begin with carbon, we notice that the carbon atom in formal charge of nitrogen of these structures shares four bonding pairs, the number of bonds typical for carbon, so it has a formal charge of zero.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. Counting electrons. About About this video Transcript. How to calculate the formal charge on nitrogen.
The concept of formal charge is actually very simple. It relates the number of electrons around an atom in a molecule's Lewis dot structure to the number of electrons that atom donated to the Lewis dot structure. In the next section we will cover drawing Lewis dot structures, and the first step is to calculate the number of electrons each atom donates to the molecule, and then to essentially draw a structure based on those electrons, placing them in either bonding or nonbonding orbitals. In formal charge calculations electrons in bonding orbitals are considered to be evenly split between the two bonding atoms, one is assigned to each atom , while those in lone pair non bonding orbitals are assigned to the atom they are placed on. A negative formal charge means there are more electrons around an atom than it donated, a positive means there are fewer electrons around an atom then it donated, and a neutral formal charge means the number it donated is the same as in the structure. The following equation determines the formal charge for each atom in a molecule or polyatomic ion.
Formal charge of nitrogen
It is more important that students learn to easily identify atoms that have formal charges of zero, than it is to actually calculate the formal charge of every atom in an organic compound. Students will benefit by memorizing the "normal" number of bonds and non-bonding electrons around atoms whose formal charge is equal to zero. A formal charge compares the number of electrons around a "neutral atom" an atom not in a molecule versus the number of electrons around an atom in a molecule.
Womens large toiletry bag
The relative contribution of non-equivalent resonance structures can be judged by a formal charge and electronegativity criterion. In a fairly uncommon bonding pattern, negatively charged nitrogen has two bonds and two lone pairs. A formal charge does not represent a true charge on an atom in a covalent bond but is simply used to predict the most likely structure when a compound has more than one valid Lewis structure. If, on the other hand, it has three bonds plus a lone pair of electrons, it will have a formal charge of So let's draw that in. A neutral nitrogen atom has five valence electrons it is in group Substituting into the formula, we obtain. A neutral hydrogen atom has one valence electron. When summed the overall charge is zero, which is consistent with the overall charge on the NH 3 molecule. Formal Charge. In this course, you will certainly need to be able to recognize whether a given species carries a charge i. We know that nitrogen is supposed to have five valence electrons, because of its position on the periodic table. Let's look at some examples of that. Nitrogen has two major bonding patterns, both of which fulfill the octet rule:.
In the previous section, we discussed how to write Lewis structures for molecules and polyatomic ions.
Why would we not assume there is one lone electron? Substituting into Equation 2. Fortunately, this ability is not terribly hard to come by — all it takes is a few shortcuts and some practice at recognizing common bonding patterns. So down here we have nitrogen. In the structures of methane, methanol, ethane, ethene, and ethyne, there are four bonds to the carbon atom. The nitrogen atom in ammonium has zero non-bonding electrons and 4 bonds. Using Equation 4. Dividing the remaining electrons between the O atoms gives three lone pairs on each atom:. Key Terms Make certain that you can define, and use in context, the key term below. In b , the sulfur atom has a formal charge of 0. The formal charges for the two Lewis electron structures of CO 2 are as follows:. In a fairly uncommon bonding pattern, negatively charged nitrogen has two bonds and two lone pairs. B We must calculate the formal charges on each atom to identify the more stable structure.

Rather quite good topic
Rather useful topic
You will not prompt to me, where to me to learn more about it?