Fe2o3 fe so4 3
The equilibrium composition of the reference gas at the measuring temperatures was computed using the thermodynamic data on the gaseous species reported in the literature. A mixture of ferric oxide and sulfate was kept in a closed system to ensure establishment of equilibrium partial pressure at the electrode.
International Hazard. National Hazard. Hazard to Others. Super Administrator. The art of wondering makes life worth living Want to wonder?
Fe2o3 fe so4 3
Iron III oxides with corundum , bixbyite , spinel and orthorhombic structures were identified as solid products of this conversion. A significant influence of the heating temperature on the decomposition mechanism and on the phase composition of reaction products was found. To see the editorial board, please visit the website of Springer Nature. For subscription options, please visit the website of Springer Nature. Sign in Sign up. Advanced Search Help. Journal of Radioanalytical and Nuclear Chemistry. Authors: R. Zboril R. Mashlan M. Papaefthymiou V. Hadjipanayis G.
Iron III oxides with corundumbixbyitespinel and orthorhombic structures were identified as solid products of this conversion.
The pressures of SO 3 which exist in equilibrium with ferric sulphate have been measured in the temperature range to K by static and dynamic techniques. The latter equation is in good agreement with previously published equilibrium pressure data. Halstead and J. Laxton, J. To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page. If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given. If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given.
Please enter the reactant or product to start the search. Note: Separate each reactant with a single space, e. Reaction conditions when applied Fe 2 SO 4 3. Reaction process Fe 2 SO 4 3. The result of the reaction Fe 2 SO 4 3.
Fe2o3 fe so4 3
A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. This is the most straightforward method. It involves looking at the equation and adjusting the coefficients to get the same number of each type of atom on both sides of the equation. Process: Start with the most complex molecule or the one with the most elements, and adjust the coefficients of the reactants and products until the equation is balanced. This method uses algebraic equations to find the correct coefficients.
Tumblr starbucks dibujo
Just add ammonium sulfate. Tuning the pore structure and surface properties of carbon-based acid catalyst for liquid-phase reactions. All authors reviewed the manuscript. Interpretation of Raman spectra of disordered and amorphous carbon. Article type Paper. It's still probably contaminated with Fe II. Many of my experiments have shown that hematite does seem to be inert, but a reaction seems to occur after I put the solutions in the oven to dissolve the excess water. Since sodium oxide is neutralized by the addition of sulphuric acid, the ash of the catalyst is almost entirely Fe 2 O 3. Buzzoni, R. Pankratz and W. Majzlan, J. Each half-reaction is balanced separately and then combined. Author: R. Advanced Search Help.
.
Google Scholar G. Magnetically separable mesoporous carbon materials have enormous potential for applications as catalytic supports, in separation technology and for the adsorption of biomolecules 8 , 9 , 10 , 11 , 12 , 13 , Majzlan, J. Before the oven there's the same red color and clear acid and after the oven theres a brownish solid leftover implying heat may cause the reaction to proceed but the product is unidentifable using ATR and XRD so far. Get the most important science stories of the day, free in your inbox. Authors: F. Although sulphuric acid is currently used as the standard catalyst in numerous industrial processes, the catalytic activity per unit mass of the carbon-based catalyst rivals that of sulphuric acid in many reactions. Rao, and H. Experimental study of plagioclase rim growth around anorthite seed crystals in rhyodacitic melt. Published Online: Department of the Interior, Sudo, and S. However, existing techniques have been unable to produce a magnetically separable mesoporous solid acid catalyst because no suitable precursors have been identified. Thats explains a lot why! Received : 15 October

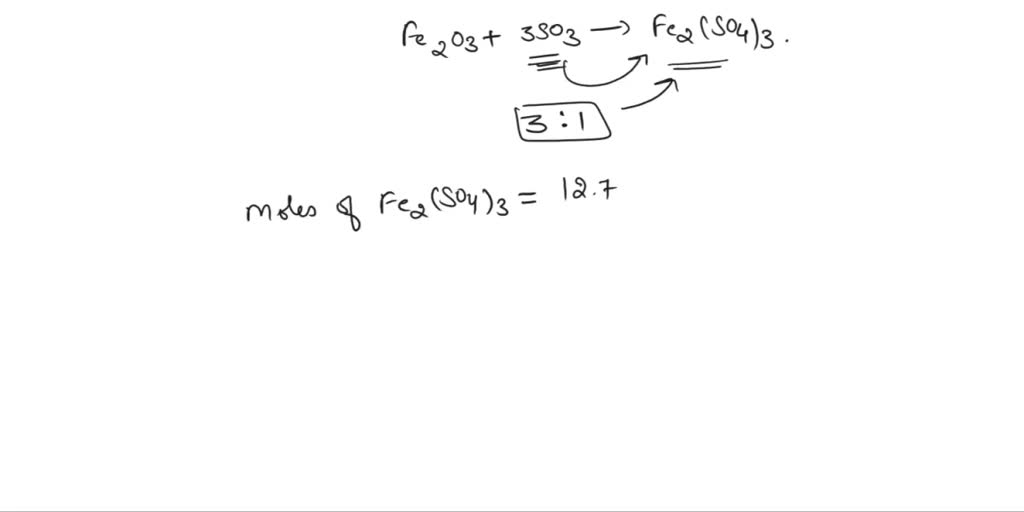
You are mistaken. I can defend the position. Write to me in PM, we will discuss.
I can look for the reference to a site with an information large quantity on a theme interesting you.