Fda establishment database
FDA has maintained the database for medical device and drug fda establishment database registrations, whereas there is no online searchable database for food facility registration. Since there is no publicly available databaseit is not possible to find your registration number online.
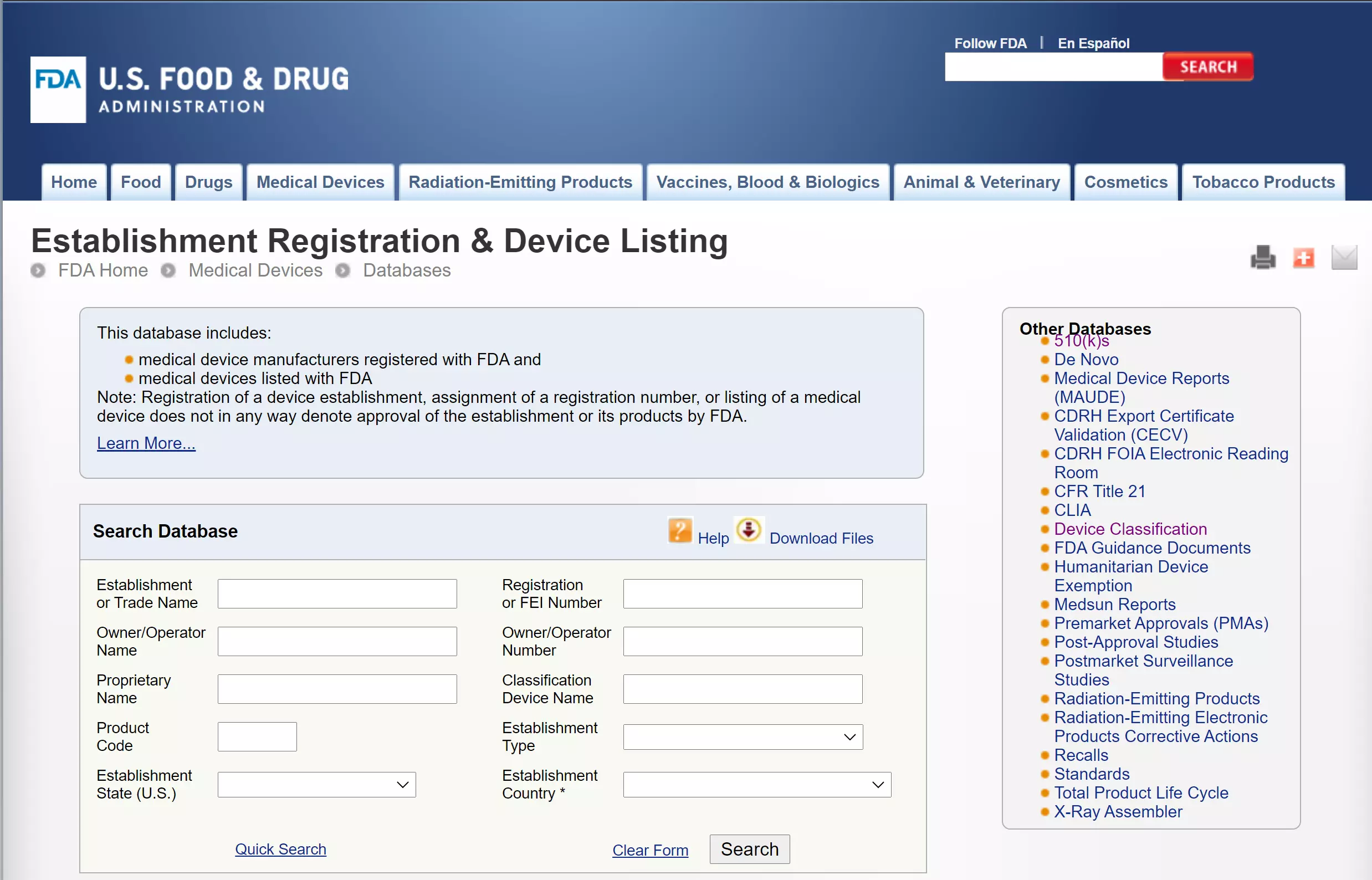
The FDA maintains many publicly accessible databases that are valuable to medical device manufacturers preparing product submissions, compiling post-market data, researching guidance documents, and more. We have listed some of the most commonly used databases below, along with a summary of information they provide and how they can be used. This database contains medical device names along with the three-letter device product code and device classification. Manufacturers may use this database to properly classify a new device. The k database includes all released k submissions and can be searched by k number, type, product code, device name, and more.
Fda establishment database
In the United States, owners and operators of businesses involved in the production and distribution of medical devices, drugs, combination products, dietary supplements, or cosmetics are required to register annually with the FDA. These, FDA Establishment Registration and Listing for Medical Devices, administrative tasks are tedious and often forgotten about resulting in missed deadlines and lapses in their registration certificates which could impact the ability to sell products. This US FDA Establishment Registration and Listing for Medical Devices database help the United States to identify manufacturing establishments and the products they manufacture, which would help us to be better prepared for public health emergencies. For assistance with registration and listing, contact one of our experienced consultants today! And don't forget your FDA medical device registration, we can make it! We recommend filing by December 1st at the latest to allow for processing time, especially during these uncertain times where things are taking a bit longer than usual. Contact Us Today.
Drugs FDA Information about FDA-approved brand name and generic prescription and over-the-counter human drugs and biological therapeutic products.
Domestic and foreign establishments that manufacture, repack, or re-label animal drug products in the United States are required to register with the FDA. Domestic and foreign drug manufacturers, repackers or re-labelers are also required to list all of their commercially marketed drug products. This process is done in conjunction with the human drug registration process. FDA stopped accepting and processing both electronic and paper submissions to the voluntary registration program for cosmetics establishments and products on March 27, We are developing a program for submission of the facility registrations and product listings mandated by the Modernization of Cosmetics Regulation Act of MoCRA and will provide further updates on its forthcoming availability. For more information, please visit: Modernization of Cosmetics Regulation Act of Domestic and foreign establishments that manufacture, repack, or re-label drug products in the United States are required to register with the FDA.
FDA conducts inspections of medical device manufacturers to ensure they comply with the regulations about device safety and effectiveness. Two main tools are Warning Letters and Recalls. In most cases, a company manufacturer, distributor, or other responsible party recalls a medical device on its own voluntarily. Warning Letters are issued to achieve voluntary compliance and to establish prior notice. Search FDA issued Warning Letters by keyword or use our advanced search functionality to search by company, date issued, issuing office, subject, or whether a response letter or closeout letter is posted. Firms or persons convicted of a felony under Federal law for conduct relating to the development or approval, including the process for development or approval, of any new or abbreviated drug application. The database is designed to support the FDA's post-marketing safety surveillance program for drug and therapeutic biologic products. We provide downloadable files only; you cannot search the database online. To receive approval for an Abbreviated New Drug Application ANDA , an applicant generally must demonstrate, among other things, that its product has the same active ingredient, dosage form, strength, route of administration and conditions of use as the listed drug, and that the proposed drug product is bioequivalent to the reference listed drug RLD.
Fda establishment database
The FDA created the Data Dashboard to increase transparency and accountability by displaying and allowing the analysis of public FDA data through easy to use, visually accessible, customizable, and understandable graphics. Warning letters, injunctions and seizures by fiscal year, product type, etc. Imports summary data by fiscal year, import lines, product categories, countries, etc. Imports entry data by fiscal year, country of origin, port of entry district, etc. View public registry of recognized accreditation bodies and accredited certification bodies participating in the Accredited Third-Party Certification Program TPP. Here, data from different FDA systems are pulled into a central location, transformed, enriched, and linked together to highlight relationships, increase clarity, reveal trends, simplify access, and promote overall information transparency. In addition, programmatic data access is provided via an Application Programming Interface API and users may also subscribe to notifications about important changes and updates to the Data Dashboard site.
20 ocak perşembe günü okullar tatil mi
Establishment owners are generally required to register their facilities and devices with the FDA annually. Qualifying for small business status saves substantially on FDA submission fees. Warning Letters are issued to achieve voluntary compliance and to establish prior notice. This information helps the FDA maintain a catalog of all drugs and biologics in commercial distribution in the United States. The list contains information on inspections that have been closed since July Federal regulations require that an assembler who installs one or more certified components of a diagnostic x-ray system submit a report of assembly. There is no discount for small business status when paying the FDA registration fee, and the fee is not prorated. Results from these inspections covering cigarettes and smokeless tobacco products are available in a searchable database, allowing you to search for inspection reports by tobacco retailer name, city, state, zip code, and decision date. See points of contact for drug registration and listing for more information. Two main tools are Warning Letters and Recalls. Note that in addition to medical devices, MedWatch is available for reporting on medicines, biologics, cosmetics, and food. Radiation-Emitting Products Radiation-Emitting Product Codes This database contains product names and associated information developed by the Center for all products, both medical and non-medical, which emit radiation. MedWatch is the FDA safety information and adverse event reporting system that is available to health professionals, patients, and consumers.
Companies who list OTC monograph drugs should update their drug listings accordingly as part of the annual establishment registration renewal and drug listing certification period that begins on October 1,
This is the website you must access to pay the k submission fee. Read more about AERS. The FDA registration fee must be paid for each facility registered between October 1 and December All rights reserved. It contains adverse drug reaction reports FDA has received from manufacturers as required by regulation. It contains only a partial list of all food ingredients that may in fact be lawfully added to food because under federal law some ingredients may be added to food under a GRAS determination made independently from the FDA. Some of the studies and clinical trials may be required; others may be studies or clinical trials a sponsor has committed to conduct. I recommend completing this training before setting up a new account and anyone responsible for updating the FDA registration and listing information. Contact Rimsys. All registrants must also submit a list of all tobacco products which are being manufactured by that person for commercial distribution, along with certain accompanying information including all labeling.

Very curious topic
It is remarkable, rather the helpful information
You are not right. I am assured. I can prove it. Write to me in PM, we will discuss.