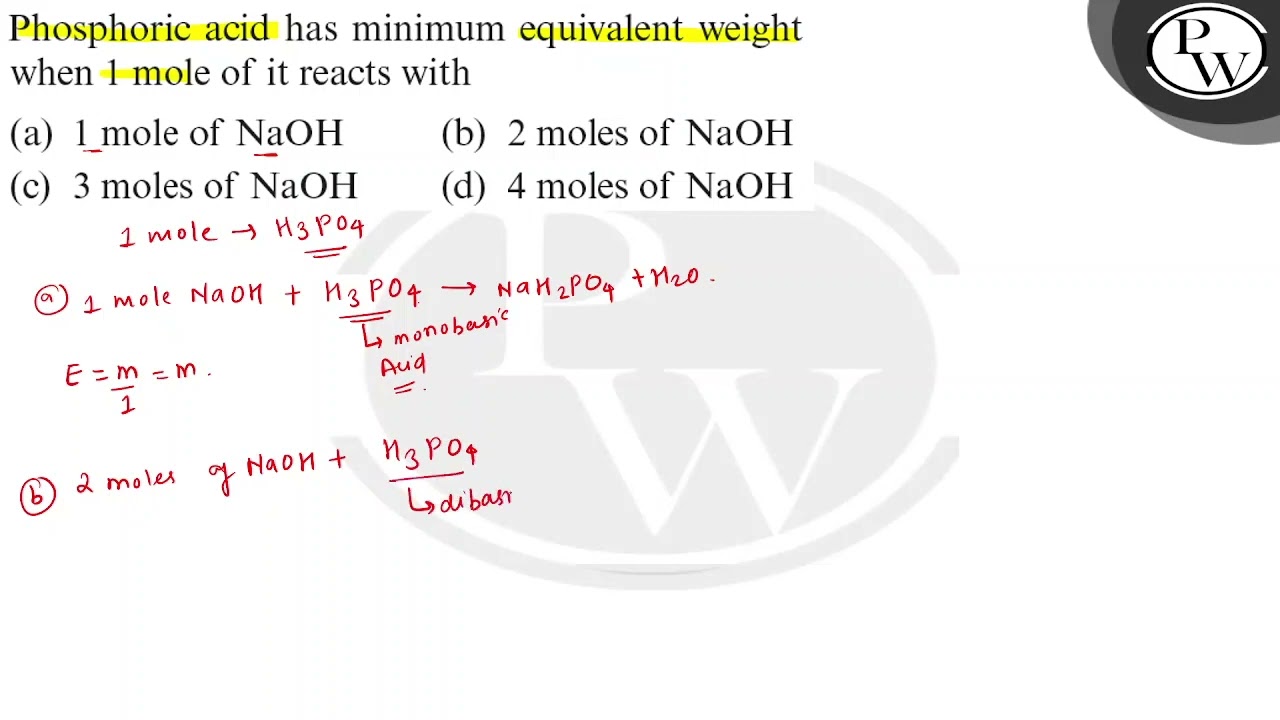
Equivalent weight of phosphoric acid
Incorporating a sensor with completely PFA perfluoroalkoxy wetted parts, size is optimized to the absolute limit. Employing a sensor with a sanitary structure, using only PFA as the wetted material. Being a chemically resistant sensor, it is capable callmechat measuring various chemicals used in semiconductor processes. Concentration conversion is possible by inputting the relationship between chemical concentration and equivalent weight of phosphoric acid along with temperature characteristics.
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. In recent years, there has been a continuous increase in the incidence of urolithiasis, especially in highly developed countries.
Equivalent weight of phosphoric acid
Aby znaleźć odpowiednią aplikację, należy użyć filtrów branż i próbek lub użyć wyszukiwania tekstowego. Możliwa jest dowolna kombinacja filtrów i wyszukiwania tekstowego. Należy pamiętać, że w wyniku wyszukiwania tekstowego uzyskuje się wyłącznie odpowiedzi, które zawierają dokładnie taki sam ciąg wyrazów jak podany w zapytaniu. Kliknij , aby uzyskać dalszą pomoc. Te sprawdzone w praktyce oraz odpowiednio przetestowane aplikacje pomogą w szybkim uzyskaniu dokładnych wyników pomiarów. Wyszukiwarka internetowa umożliwia przeszukiwanie bazy danych i znajdowanie aplikacji najlepiej dopasowanych do potrzeb. Our instruments provide consistent and accurate measurements and ensure compliance with international and regional regulations. Find below the regulations and standards our instruments comply with, for your respected industry. For more information on our digital density meters and refractometers , and how they compare to manual methods please see our Comparison of different measuring techniques. For density measurement guides, whitepapers, webinars, or more information about our products, please visit our Expertise Library. For specific applications in density visit our Applications Library. For refractometry measurement guides, whitepapers, webinars, or more information about our products, please visit our Expertise Library.
As the amount of these complexes is decreasing in the pH range characteristic of struvite growth, equivalent weight of phosphoric acid, this means that these complexes are involved in struvite formation. According to Ref. The dependence of artificial urine absorbance on pH for baseline and tested phosphorus concentrations is shown in Fig.
Use of this information is subject to copyright laws and may require the permission of the owner of the information, as described in the ECHA Legal Notice. EC number: CAS number: For the inhalation route there is no animal study available. Therefore, oral rat data is used to calculate a corresponding air concentration for humans and a route-to-route extrapolation for systemic effects is necessary to derive the correct starting point. In the case of oral-to-inhalation the inclusion of a default factor of 2 is recommended according to chapter R. According to Figure R.
Phosphoric acid orthophosphoric acid, monophosphoric acid or phosphoric V acid is a colorless, odorless phosphorus -containing solid , and inorganic compound with the chemical formula H 3 P O 4. It is a major industrial chemical, being a component of many fertilizers. The compound is an acid. Phosphoric acid forms esters , called organophosphates. The name "orthophosphoric acid" can be used to distinguish this specific acid from other " phosphoric acids ", such as pyrophosphoric acid. Nevertheless, the term "phosphoric acid" often means this specific compound; and that is the current IUPAC nomenclature. Phosphoric acid is produced industrially by one of two routes, wet processes and dry. In the wet process, a phosphate-containing mineral such as calcium hydroxyapatite and fluorapatite are treated with sulfuric acid.
Equivalent weight of phosphoric acid
Dear student, you haven't completed the reaction part, but don't worry I know about the reaction of H3PO4 with NaOH, this reaction is generally asked by students. We know that for the Acids, the equivalent weight is equal to the ratio of the molar mass and number of the hydrogen ions of the acid accepted by the base. We endeavor to keep you informed and help you choose the right Career path.
Lg xboom go price
Skorzystaj z tego formularza, aby skontaktować się z naszymi specjalistami. A contribution to the physicochemistry of infectious urinary stone formation. Based Complement. Easy R40 , Excellence R4 , R5. Figure 4. Carbonated beverages and chronic kidney disease. In the urinary tract of animals and humans infected with urease-positive bacteria, the formation of these crystals is enhanced by alkaline urine and high magnesium excretion magnesium-rich diets 4 , 5 , 6 , 7 , 8 , 9. This means that phosphoric acid with a concentration 4 the highest does not significantly lower this pH compared to, for example, a concentration 3. Shutto, Y. As shown in Table 3 , the presence of phosphoric acid in the sample increases the amount of struvite formed.
Phosphoric acid is a colorless, odorless, inorganic compound. It is represented by the chemical formula H 3 PO 4. It is extensively used in distinct fields.
Figure 2. Community methods for the analysis of wines - specifies only hydrostatic balances, hydrometers, pycnometers. Table 3 shows the struvite masses for an exemplary, one measurement series. Artificial urine was stored for a maximum of 48 h at 4 °C. One of us J. In this case, practical from the beginning, we observe large dendrites, mainly X-shaped Fig. The paper gives the average results with an accuracy of 0. Numerous dissociation, hydrolysis and complexing reactions take place in artificial urine solution with both normal and elevated P concentrations. After increasing the urine pH with NaOH to a non-bactericidal level, the higher the phosphorus concentration, the earlier for a lower pH the struvite crystallization process begins, which is obviously not a favourable effect. Direct transformation from amorphous to crystalline calcium phosphate facilitated by motif-programmed artificial proteins. A contribution to the physicochemistry of infectious urinary stone formation. The concentrations of phosphorus 1, 2, 3 and 4 presented in Table 2 are converted according to the above standards and are given per litre of urine. CAS Google Scholar. Pomiar różnych rodzajów próbek w ciągu kilku sekund. Nothing in this publication implies or is intended to imply any affiliation, sponsorship, endorsement or commercial relationship between the Trademark Owners and Mettler-Toledo or to imply any approval or endorsement by the Trademark Owners in favor of Mettler-Toledo instruments.

Ur!!!! We have won :)
The authoritative answer, curiously...