Equivalent mass of copper
Distinguish among the different physical states of matter. Define the following a equivalent mass of equivalent mass of copper acid b equivalent mass of a base c equivlent mass of an oxidising agent d equivalent mass of a reducing agent. Choose the incorrect statement.
Ask your doubts live everyday Join our live doubt clearing session conducted by our experts. Give tests to analyze your progress and evaluate where you stand in terms of your JEE preparation. The equivalent mass of chlorine is The equivalent mass of copper chloride is Hence, formula of copper chloride is. None of these. Valency of copper.
Equivalent mass of copper
Statement II : Equivalent weight of any metal is the gm quantity of metal which is combined with 8 gm of oxygen in the formation of metal oxide. Assertion : Equivalent weight of C u in C u O is The equivalent weight of iron in ferric chloride is At. If Zn has an equivalent weight of Heating mixture of C u 2 O and C u 2 S will give. A mixture of NH 3 g and N 2 H 4 g is placed in a sealed containe Chemical absorbes can be used to remove exhaled CO 2 of space travell Copper forms two oxides. For the same amount of copper, twice as much The mass of one litre sample of ozonised oxygen at NTP was found to be A sample of gaseous hydrocarbon occupying 1.
Justify the following reaction is a redox reaction. Two elements X at.
.
Forgot password? New user? Sign up. Existing user? Log in.
Equivalent mass of copper
In chemistry , equivalent weight also known as gram equivalent [1] or equivalent mass is the mass of one equivalent , that is the mass of a given substance which will combine with or displace a fixed quantity of another substance. The equivalent weight of an element is the mass which combines with or displaces 1. These values correspond to the atomic weight divided by the usual valence ; [2] for oxygen gas as example that is Equivalent weight has the units of mass, unlike atomic weight , which is now used as a synonym for relative atomic mass and is dimensionless.
Humpday significado
Why alkali metals and alkaline earth metals are called s-block elements? The equivalent weight of iron in ferric chloride is At. Determine the formula of ammonia from the following data: i Volume Give tests to analyze your progress and evaluate where you stand in terms of your JEE preparation. If Zn has an equivalent weight of Calculate the density of copper. The equivalent mass of copper chloride is Thus, per cent yield of is easy View solution. The number of moles of ethane in 60 g is. The number of moles of ethane in 60 g is Text Solution. None of these. The density of copper is. Define limiting reagent.
In the last few articles, we have studied the hydrogen displacement method, oxide formation method, reduction method, chloride formation method, and double displacement method to determine the equivalent mass of metal. The equivalent mass of a substance is the number of parts by mass of the substance which combines with or displaces or contains 1. If the equivalent mass is expressed in grams then it is called gram equivalent mass GEM.
Copper crystal has a face -centred cubic lattice structure. The equivalent mass of chlorine is Justify the following reaction is a redox reaction. The molecular formula of the compound is. Was this answer helpful? Copper crystallizes in a cubic lattice structure. Atomic radius of copper is pm and its atomic mass is Heating mixture of C u 2 O and C u 2 S will give. In the following reaction, which choice has value twice that of the equivalent mass of the oxidising agent? A steady current of Choose the incorrect statement. Its molar mass is 60 g. Hence, formula of copper chloride is. Video Solution. The equivalent mass of chlorine is

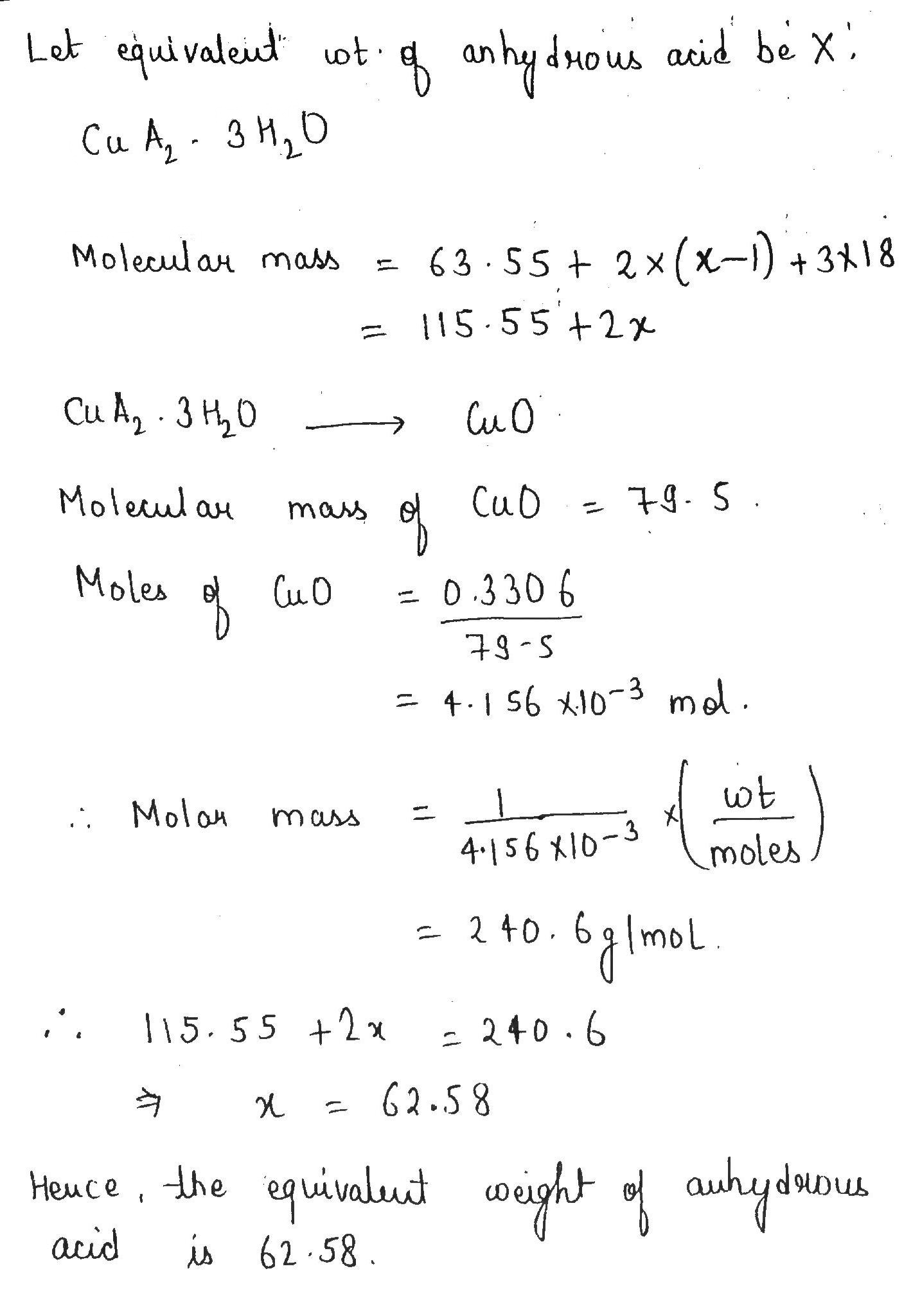
It really pleases me.
I advise to you to look a site, with a large quantity of articles on a theme interesting you.