Electron dot diagram for barium
This is a file from the Wikimedia Commons. The description on its description page there is shown below.
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot symbol or electron dot diagram or a Lewis diagram or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. It does not matter what order the positions are used. Figure 1.
Electron dot diagram for barium
Wiki User. There should be two valence electrons around the element since Strontium is in the second column of the Periodic Table and has two valence electrons filling the 5s shell. The dot structure for Rubidium is Rb with a dot on the top right of b. Rb is the short form of rubidium. The dot structure as you know can only be a max of 8 and dots are added counterclockwise. Anyway, a good counting place on the periodic table would be the second row or period. It starts with hydrogen. It and the group under it have 1 dot which is called a valence electron. As you count across, the dots increase. Beryllium has two dots. Transition metals are not counted because they can't be counter. Skip them to boron. Their group has 3 dots. Carbon has 4, Nitrogen has 5, oxygen has 6, fluorine has 7, and helium or neon have 8.
Author: Griffiths, David J. The dot structure for Rubidium is Rb with a dot on the top right of b.
To draw: the electron dot diagram for BaCl 2. A chemical bond due to the electrostatic attraction between oppositely charged ions is defined as ionic bonding. The stable configuration of any elements should contain two electrons or eight electrons in their outer shell or valence shell. The elements of barium are related to group 2. Hence, the valence electrons in these elements are 2.
Barium Lewis Dot structure would be described in this article by representing different compounds of barium. The different Lewis dot structure of different barium compounds would highlight the significant of Lewis structure in understanding their electron transfer theories. The compounds that would be drawn through Lewis structure are being listed below:. Lewis Dot structure of barium oxide shows the electron transfer process maintained in the formation of the compound. Therefore, it would be significant to draw the structure and build the idea practically. Lewis dot structure of Barium oxide is reliable in defining the nature of the bonds made by the compound, which is being represented below:. The image is denoting that the valance of Barium is two and oxygen has six valances.
Electron dot diagram for barium
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot diagram or electron dot diagram, or a Lewis diagram, or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom.
Factors of 126
How do you draw Lewis dot diagram of Br with a negative charge? Author: Griffiths, David J. Still have questions? Slater, Jeff P. So to write the Lewis structure, write the symbol Br, with 8 dots around it. Namespaces File Discussion. The material on this site can not be reproduced, distributed, transmitted, cached or otherwise used, except with prior written permission of Answers. Lewis electron dot diagrams for ions have fewer for cations or more for anions dots than the corresponding atom. The O atom has three pair of dots and the others have two pair of dots. The person who associated a work with this deed has dedicated the work to the public domain by waiving all of their rights to the work worldwide under copyright law, including all related and neighboring rights, to the extent allowed by law. Write your answer Privacy Policy. What is the formula for barium ion and hydroxide ion?
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule.
Publisher: Glencoe Mcgraw-Hill. Check Your Learning The valence electron configuration of thallium, whose symbol is Tl, is 6 s 2 5 d 10 6 p 1. CC licensed content, Shared previously. Exercises 1. What is the Lewis dot structure of the nitronium ion? How do you draw Lewis dot diagram of Br with a negative charge? Barium ion and hydroxide ion formula? Figure 2. Show Answer. Rb is the short form of rubidium.

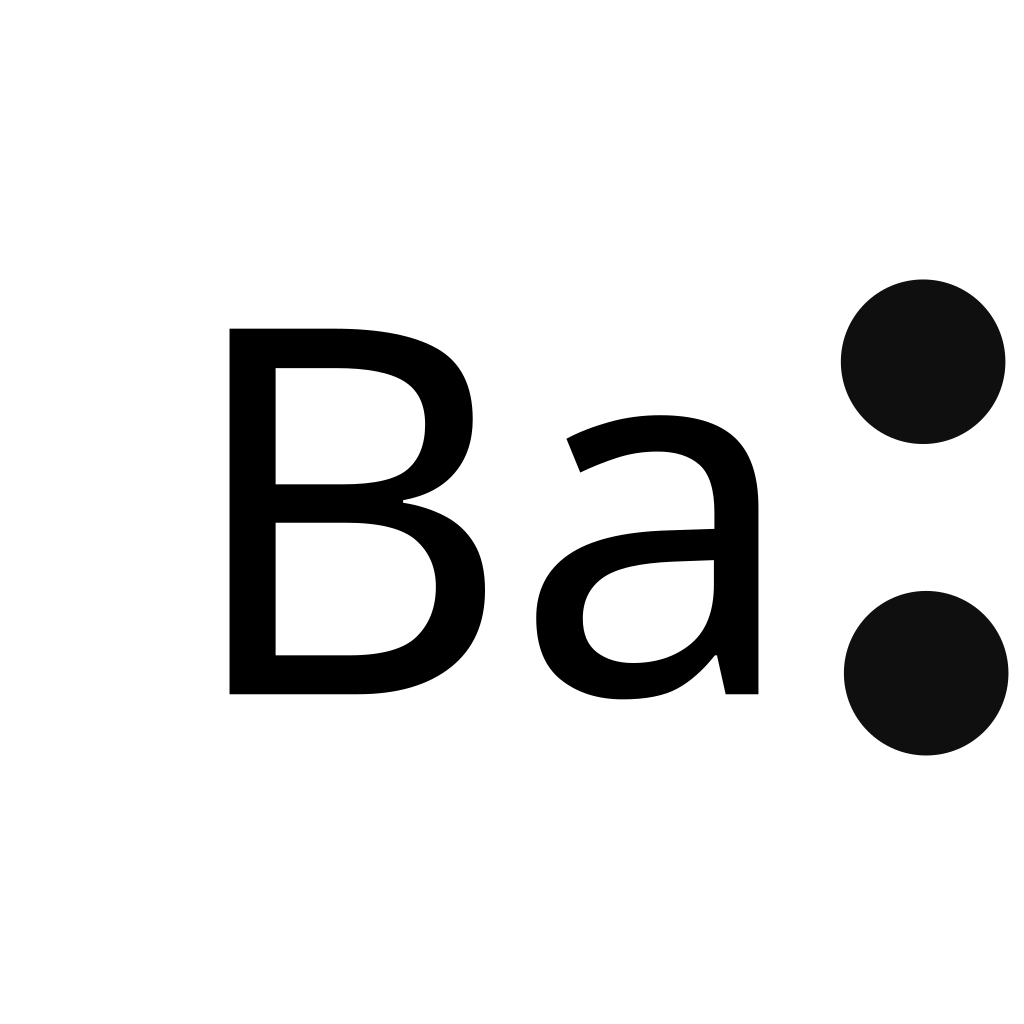
Rather valuable phrase
I congratulate, excellent idea and it is duly