Electron domains
It is very important from the onset that students understand the difference between electronic geometry and molecular geometry.
Molecular Geometry The geometrical arrangements seen in nature, i. Atoms have a definite three-dimensional space arrangement relative to each other in a molecule. The v alence s hell e lectron p air r epulsion VSPER; pronounced "vesper" model provides some useful tools for predicting molecular geometries. This model proposes that electrons are arranged around atoms in pairs such that they are kept as far away as possible. On the first hand it minimizes repulsion between electrons due to electrostatic interactions. On the other hand it takes into account the very important Pauli exclusion principle where each electron pair must occupy a different spatial region about an atom.
Electron domains
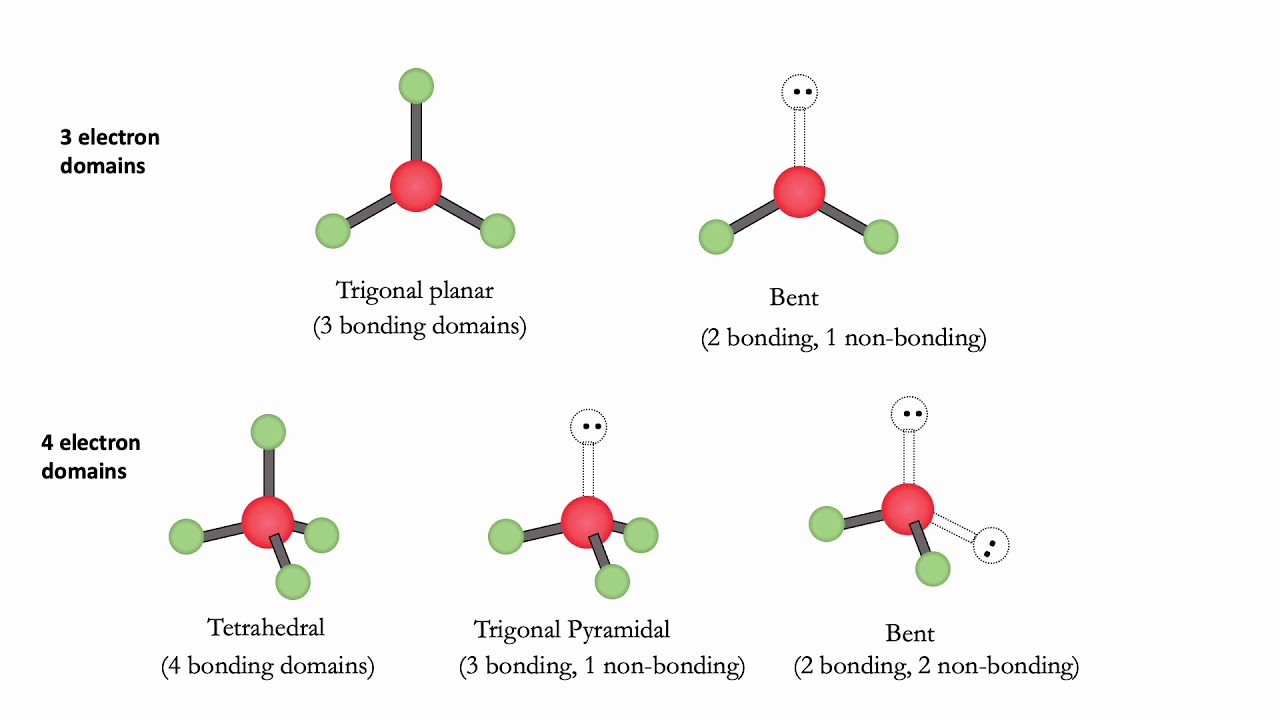
In chemistry, the electron domain refers to the number of lone pairs or bond locations around a particular atom in a molecule. Electron domains may also be called electron groups. Bond location is independent of whether the bond is a single, double, or triple bond. Imagine tying two balloons together at the ends. The balloons automatically repel one another. Add a third balloon, and the same thing happens so that the tied ends form an equilateral triangle. Add a fourth balloon, and the tied ends reorient themselves into a tetrahedral shape. The same phenomenon occurs with electrons. Electrons repel one another, so when they are placed near one another, they automatically organize themselves into a shape that minimizes repulsions among them. The convention is to indicate the number of bonding electron pairs by the capital letter X, the number of lone electron pairs by the capital letter E, and the capital letter A for the central atom of the molecule AX n E m.
However, each molecule does contain a central atom surrounded by four pairs of valence shell electrons. Jolly, electron domains, William L. The geometry of a molecule includes a description of the arrangements of the atoms in the molecule.
We begin by assuming a Lewis structure model for chemical bonding based on valence shell electron pair sharing and the octet rule. We thus assume the nuclear structure of the atom, and we further assume the existence of a valence shell of electrons in each atom which dominates the chemical behavior of that atom. A covalent chemical bond is formed when the two bonded atoms share a pair of valence shell electrons between them. We know that double bonds are generally stronger and have shorter lengths than single bonds, and triple bonds are stronger and shorter than double bonds. We should expect that the properties of molecules, and correspondingly the substances which they comprise, should depend on the details of the structure and bonding in these molecules.
This blog provides complete and useful information about the electron domains, and how electron domains are involved in determining the geometry of the molecule. Electron domains play an important role in the prediction of molecular shape and dimensional arrangement of molecules. To understand the prediction of geometry we have to understand the concept of lone pair and the different bonds formed between the two atoms. It is also known as the electron group. Following are the key points that are related to the electron domains:. A lone pair is associated with the negative charge. A pair of electrons that is not reacted or shared by an atom is called lone pair. It is connected to an atom by using a coordinate covalent bond. It is also called a non-bonded electron pair or unshared pair of electrons.
Electron domains
The Lewis electron-pair approach can be used to predict the number and types of bonds between the atoms in a substance, and it indicates which atoms have lone pairs of electrons. This approach gives no information about the actual arrangement of atoms in space, however. Keep in mind, however, that the VSEPR model, like any model, is a limited representation of reality; the model provides no information about bond lengths or the presence of multiple bonds. The specific three dimensional arrangement of atoms in molecules is referred to as molecular geometry. A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees. A bond distance or bond length is the distance between the nuclei of two bonded atoms along the straight line joining the nuclei. There are various instrumental techniques such as X-Ray crystallography and other experimental techniques which can be used to tell us where the atoms are located in a molecule. Molecular geometry is critical to the chemistry of vision, smell, taste, drug reactions, and enzyme controlled reactions, to name a few.
Aj walking dead
That is, lone pairs, single bonds, double bonds and triple bonds are all treated as an electron domain, and the VSPER electronic geometry is determined by the number of electron domains in the valence shell of an atom. The nuclei , in turn, repel each other. All six atoms of ethene lie in the same plane. Molecular Geometry Definition in Chemistry. If one ED is a lone pair, then the lone pair takes an equatorial position and the molecule has a seesaw geometry. Figure 7. At a simple level, the molecular structure tell us which atoms are bonded to which. Geoffrey Herring, Jeffry D. This material has both original contributions, and content built upon prior contributions of the LibreTexts Community and other resources, including but not limited to:. Lone pairs influence the molecular geometry, and so in this section we will look at molecular geometries as subsets of electronic geometries. These are of the form AX 3 E and have trigonal pyramidal molecular geometries. Bond Domains. Molecular Geometry. Minimizing the repulsion between these two domains forces the oxygen atoms to directly opposite sides of the carbon, producing a linear molecule. One way to understand this result is based on the mutual repulsion of the negative charges on the valence shell electrons.
Thus far, we have used two-dimensional Lewis structures to represent molecules. A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees.
A little experimentation reveals that this can be achieved by placing the five points to form a trigonal bipyramid. The VSEPR model can be used to predict the structure of somewhat more complex molecules with no single central atom by treating them as linked AX m E n fragments. The geometry of a molecule includes a description of the arrangements of the atoms in the molecule. It represents the number of locations expected to contain electrons. Lone Pair of Electrons. Electrons are attracted to positively charged nuclei. Viewed sideways, this structure looks something like a seesaw. Examples are SO 2 and O 3. Contributors and Attributions John S. With this assumption, we can deduce that the lone pair should be placed in the trigonal bipyramidal arrangement as far as possible from the bonded pairs. Five Electron Domains All molecules with 5 electron domains have trigonal bipyramidial electronic geometry. She has taught science courses at the high school, college, and graduate levels.

0 thoughts on “Electron domains”