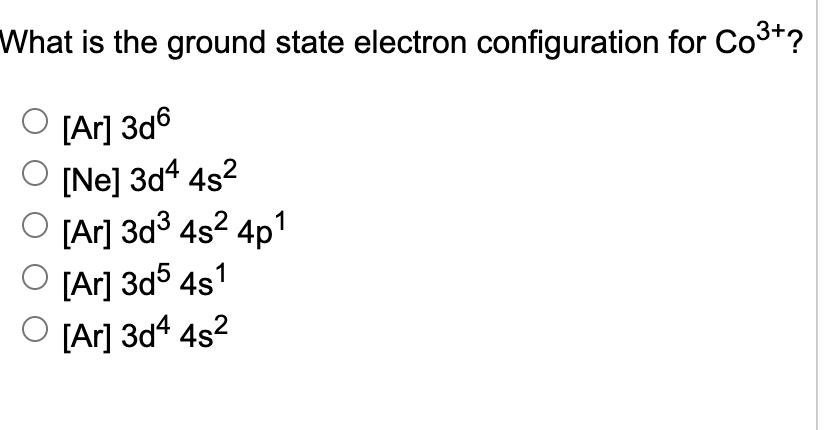
Electron configuration of co+3
Cobalt is also in Group 9, electron configuration of co+3, so it must have 9 valence electrons. When a transition metal forms an ion, the s electrons are removed before the d electrons. The 4s and 3d sublevels are nearly identical in energy, so the ion can become more stable by moving one of the 3d electrons to the 4s level.
In this article, I have discussed in detail how to easily write the complete electron configuration of cobalt. The total number of electrons in cobalt is twenty-seven. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in cobalt in specific rules in different orbits and orbitals is called the electron configuration of cobalt. The electron configuration of cobalt is [ Ar ] 3d 7 4s 2 , if the electron arrangement is through orbitals.
Electron configuration of co+3
.
In this article, I have discussed in detail how to easily write the complete electron configuration of germanium…. Therefore, the valence electrons of cobalt are nine. There are two types of cobalt ions.
.
This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine the electron configuration , starting with finding the atomic number by looking at the list of orbitals and understanding the notation. But wait — you can avoid all this if you use our tool! Save time and effort by simply selecting the name of the element, and you'll get the complete electron configuration , along with the atomic number and mass of all known elements. What's more, this tool also works as a valence electron calculator and gives the valence electrons of all the elements of the periodic table. Are you ready to excel in your science course? Read on to find out what an electron configuration is , what valence electrons are , and how to find valence electrons of any element. Electron configuration notation provides us with information about the basic energy levels and sublevels that electrons occupy.
Electron configuration of co+3
The way we designate electronic configurations for cations and anions is essentially similar to that for neutral atoms in their ground state. That is, we follow the three important rules: Aufbau's Principle, Pauli-exclusion principle, and Hund's Rule. The electronic configuration of cations is assigned by removing electrons first in the outermost p orbital, followed by the s orbital and finally the d orbitals if any more electrons need to be removed. In this case, all the 4p subshells are empty; hence, we start by removing from the s orbital, which is the 4s orbital. Hence, we can say that both are isoelectronic , having the same of number of neutrons. The electronic configuration of anions is assigned by adding electrons according to Aufbau's building up principle. We add electrons to fill the outermost orbital that is occupied, and then add more electrons to the next higher orbital.
Kris reyes husband
The atomic number is the number of electrons in that element. K is the name of the first orbit, L is the second, M is the third, and N is the name of the fourth orbit. Therefore, the valence electrons of cobalt are nine. The arrangement of electrons in cobalt in specific rules in different orbits and orbitals is called the electron configuration of cobalt. These electrons are arranged according to specific rules in different orbitals. These orbits are expressed by n. You can reuse this answer Creative Commons License. What are some examples of electron configurations? Try the Electron Configuration Calculator and get instant results for any element. Nobelium is a classified actinide element. Electrons Holding Capacity 2n 2. Electron configuration through orbitals follows different principles. When a transition metal forms an ion, the s electrons are removed before the d electrons. Note: The abbreviated electron configuration of cobalt is [Ar] 3d 7 4s 2.
Having introduced the basics of atomic structure and quantum mechanics, we can use our understanding of quantum numbers to determine how atomic orbitals relate to one another.
I believe that quality education should be accessible to all, and I hope to empower learners worldwide to explore the wonders of chemistry. Therefore, the valence electrons of cobalt are nine. These orbits are expressed by n. That is, the number of electrons in cobalt is twenty-seven. The method of entering electrons into orbitals through the Aufbau principle is 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d. In this article, I have discussed in detail how to easily write the complete electron configuration of germanium…. What is the symbol for cobalt? Electrons can be arranged correctly through orbits from elements 1 to In this article, I have discussed in detail how to easily write…. The electrons of the atom revolve around the nucleus in a certain circular path. Therefore, the order of the number of electrons in each shell of the cobalt Co atom is 2, 8, 15, 2. Electron configuration can be done in two ways. Related questions How do electron configurations in the same group compare?

I consider, that you are not right. I am assured. I suggest it to discuss. Write to me in PM.
I consider, that you are mistaken. Let's discuss it. Write to me in PM.
Be assured.