Draw the lewis structure for acetic acid
Cheers for this great post. I believe that you have raised some interesting poinst here.
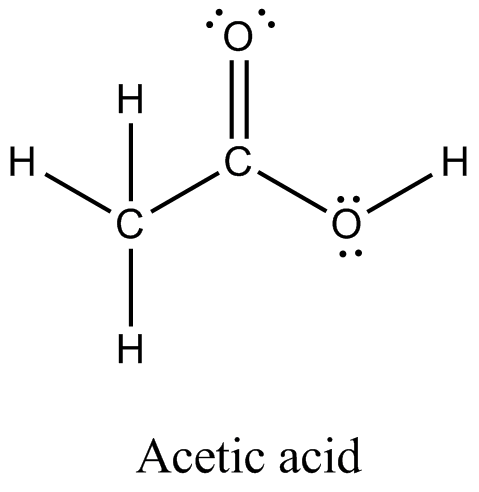
There are 2 lone pairs on both the Oxygen atoms O. In order to find the total valence electrons in a CH3COOH molecule , first of all you should know the valence electrons present in carbon atom , hydrogen atom as well as oxygen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Carbon is group 14 element on the periodic table. Hydrogen is group 1 element on the periodic table.
Draw the lewis structure for acetic acid
Each carbon atom is also bonded to three hydrogen H atoms, and the remaining oxygen atom has two lone pairs of electrons. Valence electrons are the outermost electrons in an atom that are involved in chemical bonding. To determine the number of valence electrons in hydrogen sulfide , add the number of valence electrons in each atom. Hydrogen has one valence electron, while oxygen has six valence electrons, and carbon has four electrons. The organic molecule is formed by a carbon chain and oxygen are attached to one carbon. The remaining oxygen atoms are bonded 3 to one carbon and one to a oxygen atom. Hydrogen can only form one bond, while carbon can form four bonds, and oxygen up to two bonds. Draw the carbon chain, then put the oxygens and finally the hydrogens. Connect 3 hydrogen atoms to the carbon atom with a single bond and another to one oxygen. Place the remaining six valence electrons around the oxygen atoms in pairs.
In other way you can also see that the carbon atom is surrounded with three hydrogen atoms and one COOH group. Due to its polar qualities it is found as a liquid at standard temperature and pressure in relation to its rather light molar mass.
.
In this comprehensive guide, we will take you through the process of drawing the Lewis structure for CH3COOH, also known as acetic acid, a molecule with significant importance in organic chemistry and beyond. Find the Total Valence Electrons. Carbon C contributes 4 valence electrons, hydrogen H has 1 valence electron, and oxygen O contributes 6 valence electrons each. Calculate the total valence electrons as follows:. Select the Central Atom. Remember : If hydrogen is present in the molecule, always place the hydrogen atoms on the outside. Then connect the first central carbon atom to each of the three hydrogen atoms using single bonds electron pairs. Connect the second central carbon atom to each of the two oxygen atoms and one hydrogen atom, respectively. Each hydrogen atom now has 2 electrons 1 in the bond and 1 as a lone pair , satisfying the duet rule for hydrogen. However, the central carbon atom and two oxygen atoms require additional electrons for stability.
Draw the lewis structure for acetic acid
Each carbon atom is also bonded to three hydrogen H atoms, and the remaining oxygen atom has two lone pairs of electrons. Valence electrons are the outermost electrons in an atom that are involved in chemical bonding. To determine the number of valence electrons in hydrogen sulfide , add the number of valence electrons in each atom. Hydrogen has one valence electron, while oxygen has six valence electrons, and carbon has four electrons. The organic molecule is formed by a carbon chain and oxygen are attached to one carbon. The remaining oxygen atoms are bonded 3 to one carbon and one to a oxygen atom. Hydrogen can only form one bond, while carbon can form four bonds, and oxygen up to two bonds. Draw the carbon chain, then put the oxygens and finally the hydrogens. Connect 3 hydrogen atoms to the carbon atom with a single bond and another to one oxygen.
Oreilly auto parts stockton ca
You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. Unfortunately, one of the carbon atoms is not forming an octet here. If we compare the electronegativity values of carbon C and oxygen O then the carbon atom is less electronegative. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Now, you can see the electronegativity values of carbon atom C and oxygen atom O in the above periodic table. As you can see every single element has a filled valence shell with the two oxygen's each containing two lone pairs of electrons, the only instance of this phenomena within the Lewis Structure. It is frequently utilized as a polar solvent in lab research settings and thereby has been involved in certain medical practices. In order to find the total valence electrons in a CH3COOH molecule , first of all you should know the valence electrons present in carbon atom , hydrogen atom as well as oxygen atom. He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations. Scroll to Top. Hacker News. You can see the number of bonding electrons and nonbonding electrons for each atom of CH3COOH molecule in the image given below. Hydrogen can only form one bond, while carbon can form four bonds, and oxygen up to two bonds. There are 2 lone pairs on both the Oxygen atoms O.
Both the oxygen atoms have 2 lone pairs.
Scroll to Top. Hydrogen has one valence electron, while oxygen has six valence electrons, and carbon has four electrons. You could alternatively also draw the structure by including two dots for every bond. Copy link. The organic molecule is formed by a carbon chain and oxygen are attached to one carbon. Connect 3 hydrogen atoms to the carbon atom with a single bond and another to one oxygen. As mentioned above acetic acid is also present in vinegar which has a variety of household uses; however, the acetic acid is diluted in water to a greater degree than in a research lab. The formal charge of an atom should be as close to zero as possible. Lone pairs are non-bonding pairs of electrons. It is frequently utilized as a polar solvent in lab research settings and thereby has been involved in certain medical practices. The Greek Philosopher Theophrastus. Oxygen is group 16 element on the periodic table. The stability of lewis structure can be checked by using a concept of formal charge.

Your phrase simply excellent