Do ionic compounds dissolve in water
We have learned that solutions can be formed in a variety of combinations using solids, liquids, and gases. We also know that solutions have constant composition, and that this composition can be varied up zooni a point to maintain the homogeneous nature of the solution. But how exactly do solutions form? Why is it that oil and water will not form a solution, do ionic compounds dissolve in water, and yet vinegar and water will?
Ionic compounds are those composed of oppositely charged atoms, called ions, arranged in a lattice structure. Salts, including sodium chloride NaCl — table salt —are the best-known examples of ionic compounds. When you immerse an ionic compound in water, the ions are attracted to the water molecules, each of which carries a polar charge. If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the compound dissolves. When this happens, the ions dissociate and disperse in solution, each surrounded by water molecules to prevent it from recombining. The resultant ionic solution becomes an electrolyte, which means it can conduct electricity.
Do ionic compounds dissolve in water
When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. These substances constitute an important class of compounds called electrolytes. Substances that do not yield ions when dissolved are called nonelectrolytes. If only a relatively small fraction of the dissolved substance undergoes the ion-producing process, it is called a weak electrolyte. Substances may be identified as strong, weak, or nonelectrolytes by measuring the electrical conductance of an aqueous solution containing the substance. To conduct electricity, a substance must contain freely mobile, charged species. Most familiar is the conduction of electricity through metallic wires, in which case the mobile, charged entities are electrons. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. The electrostatic attraction between an ion and a molecule with a dipole is called an ion-dipole attraction. These attractions play an important role in the dissolution of ionic compounds in water. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. This process represents a physical change known as dissociation. Under most conditions, ionic compounds will dissociate nearly completely when dissolved, and so they are classified as strong electrolytes. Let us consider what happens at the microscopic level when we add solid KCl to water.
We also know that solutions have constant composition, and that this composition can be varied up to a point to maintain the homogeneous nature of the solution. Outside of this cluster of spheres are seventeen clusters of three spheres, which include one red and two white spheres.
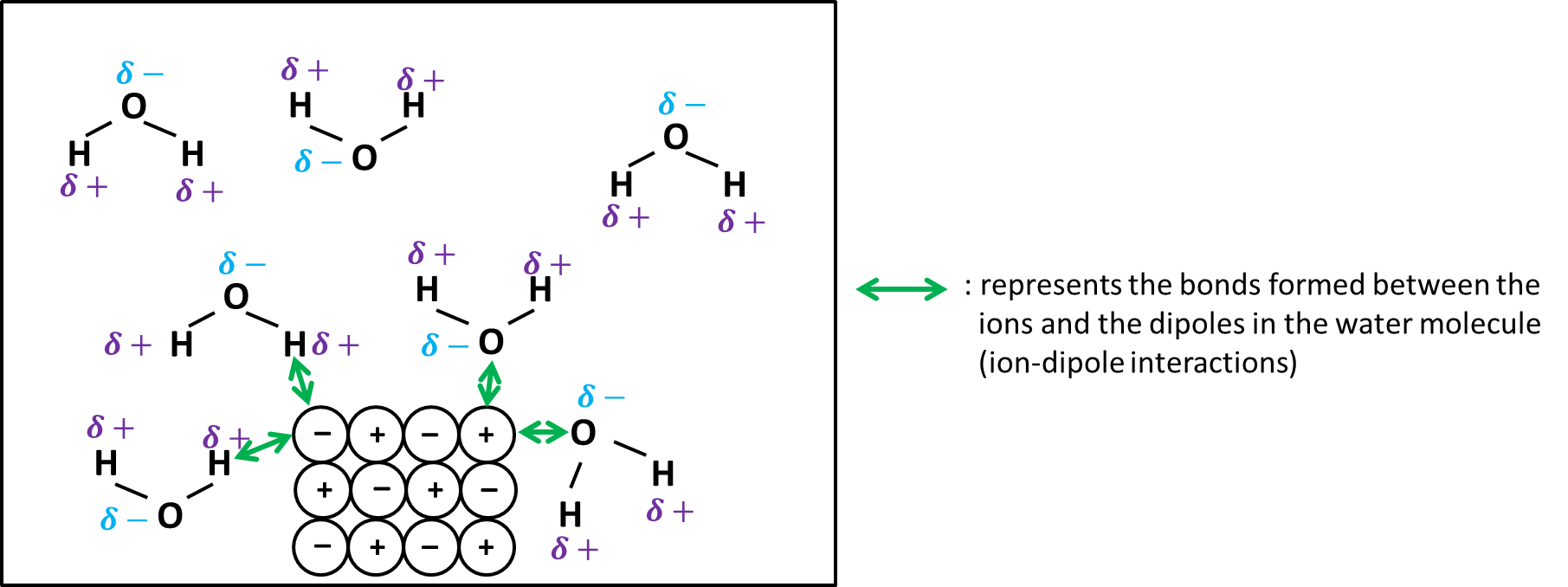
To dissolve an ionic compound, the water molecules must be able to stabilize the ions that result from breaking the ionic bond. The "O" atom has a partial negative charge, and the "H" atoms have a partial positive charge. When you place an ionic substance in water, the water molecules attract the positive and negative ions from the crystal. The positive ions have several water molecules around them, all with their "O" atoms close to the positive ion. The negative ions have several water molecules around them, all with their "H" atoms close to the negative ion.
A simple ionic compound, such as sodium chloride NaCl consists of a sodium cation and a chloride anion. Because these are oppositely charge ions, they are strongly attracted to each other. This attraction is non-specific and the sodium cation would also be strongly attracted to any anion. When an ionic compound dissolves in water, the individual cations and anions are completely surrounded by water molecules, but these water molecules are not randomly oriented. A sodium cation in water will be surrounded by water molecules oriented so that the negative end of the molecular dipole is in contact with the sodium cation. Likewise, the waters surrounding the chloride anion are oriented so that the positive end of the molecular dipole contacts the anion. When arranged like this, the charged poles of the water molecules neutralize , and thus stabilize the charges on the ions. The ability of water to interact with and stabilize charge particles goes well beyond the water molecules that actually touch the ion.
Do ionic compounds dissolve in water
Water-soluble ionic compounds such as acids, bases, and salts dissociate in water forming ions. In fact, this interaction between the ions and water molecules is the driving force for dissolving and ionizing the salt. The first thing you will need is to determine whether the compound is soluble or not. If it is not, then you cannot write a dissociation equation for it because it only dissociates when dissolved in water. To determine this, use the following rules for different combinations of cations and anions that make the salt:. The perchlorate ion ClO 4 — is listed as soluble with any cation without exceptions. Therefore, it is a soluble salt.
Imaging matlab
Go back to previous article. These substances constitute an important class of compounds called electrolytes. Its positive end is attracted to the negative ions in an ionic compound, while the negative end is attracted to the positive ions. Solubility curves can be used to determine if a given solution is saturated or unsaturated. When the maximum amount of solute has been dissolved in a given amount of solvent, we say that the solution is saturated with solute. The white spheres of these clusters are closest to the purple spheres. All chlorides, bromides, and iodides. The other end leads to a rectangle labeled with a minus sign. The positive and negative ions drift off into the solution. This is why athletes prefer electrolytic drinks to pure water.
Substances may be identified as strong, weak, or nonelectrolytes by measuring the electrical conductance of an aqueous solution containing the substance. To conduct electricity, a substance must contain freely mobile, charged species.
Question ae. Water is a polar molecule. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. All carbonates and phosphates. Substances that do not yield ions when dissolved are called nonelectrolytes. Every ion is a spectator ion and there is no net ionic equation at all. Why do ionic compounds dissolve in water? This process represents a physical change known as dissociation. All chlorides, bromides, and iodides. The reduction of the electrostatic attraction permits the independent motion of each hydrated ion in a dilute solution, resulting in an increase in the disorder of the system, as the ions change from their fixed and ordered positions in the crystal to mobile and much more disordered states in solution. The ions are hydrated. Solutions of electrolytes contain ions that permit the passage of electricity. Related questions Question 79c2f.

You will not prompt to me, where to me to learn more about it?
I consider, that you commit an error. I suggest it to discuss.
It is a valuable phrase