Diacylglycerol
The neutral lipids diacylglycerols DAGs are involved in diacylglycerol plethora of metabolic pathways, diacylglycerol. They function as components of cellular membranes, as building blocks for glycero phospho lipids, and as lipid second messengers.
Lipids in Health and Disease volume 19 , Article number: Cite this article. Metrics details. DAG accumulates in multiple organs of the obese subjects, which leads to the disruption of metabolic homeostasis and the development of diabetes as well as associated diseases. Multiple studies proved that aberrant activation of PKCs and PKDs contributes to the development of metabolic diseases. DAG-sensing PKC and PKD isoforms play a crucial role in the regulation of metabolic homeostasis and therefore might serve as targets for the treatment of metabolic disorders such as obesity and diabetes.
Diacylglycerol
Federal government websites often end in. The site is secure. The neutral lipids diacylglycerols DAGs are involved in a plethora of metabolic pathways. They function as components of cellular membranes, as building blocks for glycero phospho lipids, and as lipid second messengers. Considering their central role in multiple metabolic processes and signaling pathways, cellular DAG levels require a tight regulation to ensure a constant and controlled availability. Interestingly, DAG species are versatile in their chemical structure. Recent scientific advances have revealed that DAG metabolizing enzymes generate and distinguish different DAG isoforms, and that only one DAG isoform holds signaling properties. Herein, we review the current knowledge of DAG stereochemistry and their impact on cellular metabolism and signaling. Further, we describe intracellular DAG turnover and its stereochemistry in a 3-pool model to illustrate the spatial and stereochemical separation and hereby the diversity of cellular DAG metabolism. For a long time, diacylglycerol DAG has been recognized as lipid molecule which exhibits signaling function. Thus, the stereochemical nature of DAG isomers by itself is a determinant for its physiological role in distinct cellular compartments and metabolic pathways.
Mol Pharmacol — Ikeda Y, et al. Diacylglycerol up for Nature Briefing.
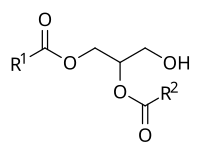
Diacylglycerols or "diglycerides" are esters of the trihydric alcohol glycerol in which two of the hydroxyl groups are esterified with long-chain fatty acids. They can exist in three stereochemical forms see our web document on Triacylglycerols part 1 for a discussion of nomenclature. Diacylglycerols are formed in animal and plant tissues as intermediates in the biosynthesis of triacylglycerols and other glycerolipids and during the hydrolysis of these by lipases. While their presence is of technological relevance in commercial seed oils as small amounts can have a profound influence on the physical properties, the biological function of sn -1,2-diacylglycerols derived from phospholipids as signalling mediators in animal tissues is of special importance for human health and wellbeing. They are the only one of the three stereoisomers that function in this way, and they are synthesised and metabolized by innumerable enzymes at spatially different cellular locations, each with distinct enzymatic properties and selectivities.
Diacylglycerols or "diglycerides" are esters of the trihydric alcohol glycerol in which two of the hydroxyl groups are esterified with long-chain fatty acids. They can exist in three stereochemical forms see our web document on Triacylglycerols part 1 for a discussion of nomenclature. Diacylglycerols are formed in animal and plant tissues as intermediates in the biosynthesis of triacylglycerols and other glycerolipids and during the hydrolysis of these by lipases. While their presence is of technological relevance in commercial seed oils as small amounts can have a profound influence on the physical properties, the biological function of sn -1,2-diacylglycerols derived from phospholipids as signalling mediators in animal tissues is of special importance for human health and wellbeing. They are the only one of the three stereoisomers that function in this way, and they are synthesised and metabolized by innumerable enzymes at spatially different cellular locations, each with distinct enzymatic properties and selectivities. The reverse reaction in which phosphatidic acid is produced by the action of a diacylglycerol kinase is likewise of great biological relevance see below.
Diacylglycerol
Published on: February 28, Promising treatment in clinical trials has double action against triglyceride and fatty acid synthesis, UTSW study shows. Their findings, published in Cell Metabolism , shed light not only on the mechanism behind this experimental drug but also on how the body normally regulates triglyceride and fatty acid production.
Hotel boutique dubai acapulco telefono
Skip to main content. The temperature and pressure were controlled by coupling the system to a stochastic velocity rescaling thermostat 53 and a Parrinello—Rahman barostat 54 , respectively. On the other hand, there are spatial isomers stereoisomers which display same linkage of atoms and functional groups but differ in their geometrical position in space. ATGL knockout mice exhibit enlarged adipose tissues. Other isoforms, like sn -2,3 or rac -1,3 DAGs, are unable to activate PKCs [ — ] and hence display no signaling properties. They can exist in three stereochemical forms see our web document on Triacylglycerols part 1 for a discussion of nomenclature. Zimmermann R, Haemmerle G, Wagner EM et al Decreased fatty acid esterification compensates for the reduced lipolytic activity in hormone-sensitive lipase-deficient white adipose tissue. Steinberg SF Structural basis of protein kinase C isoform function. ASO injection into mice mainly targets the liver, adipose tissues, and kidney and does not lead to a global silencing of expression. Positional specificity of hormone-sensitive lipase from rat adipose tissue. Osterlund T, Contreras JA, Holm C Identification of essential aspartic acid and histidine residues of hormone-sensitive lipase: apparent residues of the catalytic triad. Biochem J, The utilization of the alpha-glycerophosphate and monoglyceride pathways for phosphatidyl choline biosynthesis in the intestine.
As both lipids are thought to function as bioactive lipid signaling molecules with distinct cellular targets, DGK therefore occupies an important position, effectively serving as a switch by terminating the signalling of one lipid while simultaneously activating signalling by another. In bacteria , DGK is very small 13 to 15 kDa membrane protein which seems to contain three transmembrane domains. Some Gram-positive bacteria also encode a soluble diacylglycerol kinase capable of reintroducing DAG into the phospholipid biosynthesis pathway.
About this article. On the other hand, the deletion of PKD1 specifically in adipocytes protects the development of od liver steatosis evoked by HFD feeding [ 79 ]. In case of TAG, the potential achiral carbon atom at sn -2 position becomes a chiral center by removal of the attached FA either at sn -1 or at sn -3 position. The core is strictly assembled by hydrophobic lipid esters, like TAG and cholesteryl esters CEs , and the surface is formed by a PL monolayer [ 3 ]. It is known that inflammation plays an important role in the pathogenesis and progression of skeletal muscle disorders, for instance, insulin resistance and Duchenne Muscular Dystrophy DMD. Azelastine MK Morley N, Kuksis A Positional specificity of lipoprotein lipase. Annu Rev Pharmacol Toxicol — Specifications of molecular chirality. Diacylglycerol kinases: at the hub of cell signalling. Inhibition of PKDs decreases myotube fusion and myoblast differentiation in primary mouse satellite cells and C2C12 stable cell line [ ]. Copy Download.

I can speak much on this theme.