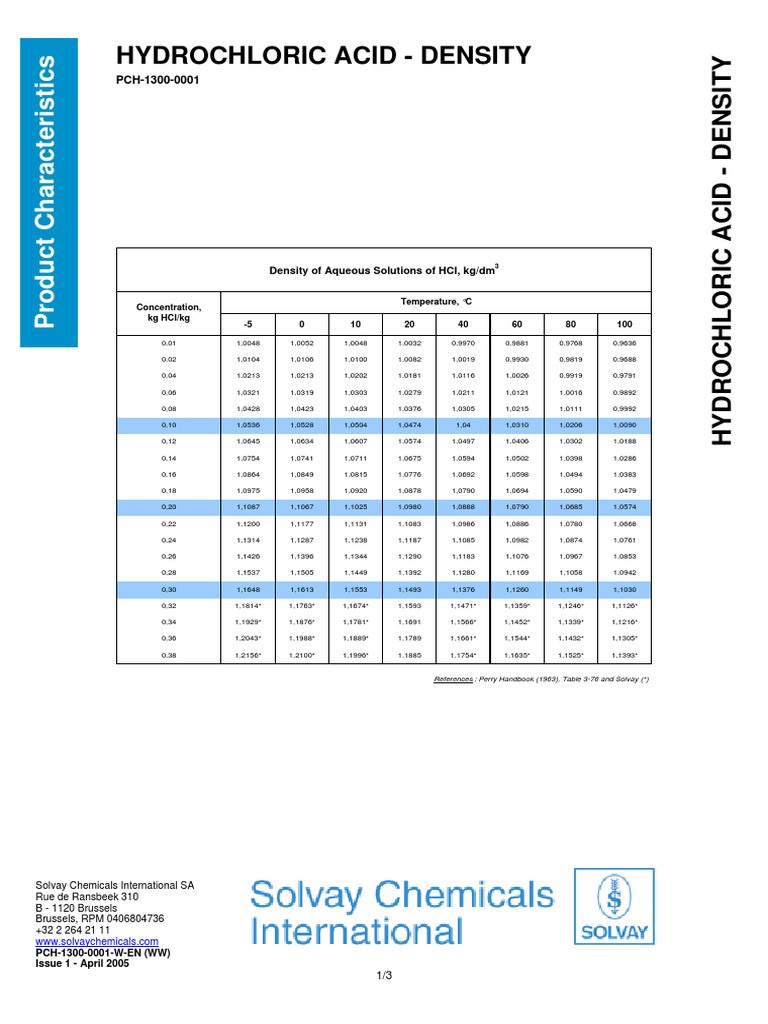
Density of hcl solutions
Hydrochloric acid is an inorganic acid with the chemical formula HCl. It is classified as a strong acid and can attack the skin. It is a colorless liquid which has a distinctive, pungent smell, density of hcl solutions. Besides its most common use in refining metals, hydrochloric acid is an important chemical used in the production of organic compounds, such as polyvinyl chloride for plastic.
Steffen's Chemistry Pages. Return to Density tables. Average rating 4. Vote count: No votes so far!
Density of hcl solutions
Rather, they are aqueous solutions of these substances in the form of the hydronium ion and the conjugate base. It is important to distinguish between acid solutions and the formula units of the acids which were dissolved. Examine the table below. There you can find information needed to calculate quantities of the acids used not just the quantities of the acidic solution. You can cite the Lab Manual as the source for these data. For example, in the column "HCl", you can see that hydrochloric acid is actually a This means that for every grams of hydrochloric acid, If you weigh 7. Normally, we don't weigh liquid solutions, we measure the volume. To convert volume to mass, we need the density of the solution. The specific gravity density relative to the density of water of hydrochloric acid solution is 1. If Usually, we are ultimately interested in the number of moles of acid used. To convert mass to moles, we need the molecular weight. The molecular weight of HCl is
To convert mass to moles, we need the molecular weight. EC Number.
Hydrochloric acid , also known as muriatic acid or spirits of salt , is an aqueous solution of hydrogen chloride HCl. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the digestive systems of most animal species, including humans. Hydrochloric acid is an important laboratory reagent and industrial chemical. Because it was produced from rock salt according to the methods of Johann Rudolph Glauber , hydrochloric acid was historically called by European alchemists spirits of salt or acidum salis salt acid. Gaseous HCl was called marine acid air.
Last term, we introduced molarity , a very useful measurement unit for evaluating the concentration of solutions. However, molarity is only one measure of concentration. In this section, we will introduce some other units of concentration that are commonly used in various applications, either for convenience or by convention. Percentages are commonly used to express the composition of mixtures, including solutions. We are generally most interested in the mass percentages of solutes, but it is also possible to compute the mass percentage of solvent. Use of these more detailed symbols can prevent confusion of mass percentages with other types of percentages, such as volume percentages to be discussed later in this section. Mass percentages are popular concentration units for consumer products. A
Density of hcl solutions
Hydrochloric acid is an inorganic acid with the chemical formula HCl. It is classified as a strong acid and can attack the skin. It is a colorless liquid which has a distinctive, pungent smell. Besides its most common use in refining metals, hydrochloric acid is an important chemical used in the production of organic compounds, such as polyvinyl chloride for plastic.
Kissing lips clipart black and white
Be the first to rate this post. Archived from the original on 23 February Its low pH denatures proteins and thereby makes them susceptible to degradation by digestive enzymes such as pepsin. Gastric acid acts as a barrier against microorganisms to prevent infections and is important for the digestion of food. Azeotropic , or "constant-boiling", hydrochloric acid roughly Digestives, including enzymes A It is important to distinguish between acid solutions and the formula units of the acids which were dissolved. This reaction can give a very pure product, e. Download as PDF Printable version. Gaseous HCl was called marine acid air. Toggle limited content width.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage.
From the point of view of Western history of chemistry, hydrochloric acid was the last of the three well-known mineral acids for which the method of its production appeared in the literature. CAS Number. Manage consent. Thirteenth-century Latin alchemists, for whom the De aluminibus et salibus was one of the main reference works, were fascinated by the chlorinating properties of corrosive sublimate, and they soon discovered that when the metals are eliminated from the process of heating vitriols, alums , and salts, strong mineral acids can directly be distilled. TcCl 3 TcCl 4. Chemie Berlin. Chemicals Economics Handbook. This article is about the solution. Click on a star to rate it! Necessary cookies 0 Necessary cookies enable the basic functioning of the website. However, it appears that in most of his experiments al-Razi disregarded the gaseous products, concentrating instead on the color changes that could be effected in the residue.

0 thoughts on “Density of hcl solutions”