Daniell cell class 12
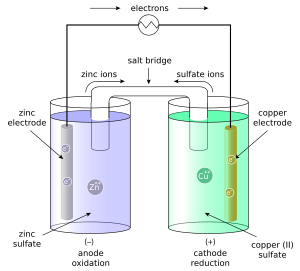
A Daniell cell is the best example of a galvanic cell which converts chemical energy into electrical energy. The Daniell cell consists of two electrodes of dissimilar metals, Zn and Cu; each electrode is in contact with a solution of its own ion; Zinc sulphate and copper sulphate respectively, daniell cell class 12.
The Daniell cell is a type of electrochemical cell invented in by John Frederic Daniell , a British chemist and meteorologist , and consists of a copper pot filled with a copper II sulfate solution, in which is immersed an unglazed earthenware container filled with sulfuric acid and a zinc electrode. He was searching for a way to eliminate the hydrogen bubble problem found in the voltaic pile , and his solution was to use a second electrolyte to consume the hydrogen produced by the first. Zinc sulfate may be substituted for the sulfuric acid. The Daniell cell was a great improvement over the existing technology used in the early days of battery development. A later variant of the Daniell cell called the gravity cell or crowfoot cell was invented in the s by a Frenchman named Callaud and became a popular choice for electrical telegraphy. The Daniell cell is also the historical basis for the contemporary definition of the volt , which is the unit of electromotive force in the International System of Units. The definitions of electrical units that were proposed at the International Conference of Electricians were designed so that the electromotive force of the Daniell cell would be about 1.
Daniell cell class 12
In this article, you will learn in detail about Daniell cell, its definition, construction, the chemical reaction involved, and its applications. Read on for more. A Daniell cell is a type of electrochemical cell that consists of a copper pot that is filled with a solution of copper II sulphate. An unglazed earthenware is immersed in this solution containing sulphuric acid and a zinc electrode. The Daniell cell was invented, while the chemist was seeking a way to eliminate the hydrogen bubble issue found in the voltaic pile. The Daniell cell was seen as good progress in battery development, if we consider the technology of those times. The Daniell cell can be defined as a primary voltaic cell that has sulphuric acid separated by a porous barrier from a copper electrode in sulphate solution. The Daniell cell is an improved version of the voltaic cell. The polarisation issue faced in a voltaic cell is addressed in a Daniell cell. The Daniell cell is basically a container made of copper that contains a concentrated solution of copper sulphate. There is one more container inside this copper container.
Image to be added soon. The zinc anode is dipped in Zinc salt solution and the Copper cathode is dipped in the copper salt solution.
Padma Priya and Laboratory Assistant, Mr. Suresh for helping me complete this project. I thank the principal of our school, Mrs. T for providing us with a well equipped laboratory for carrying out our experiments. I would like to thank my parents for supporting my work on this project. One of their oldest and most simple incarnation was the Daniell cell.
Access premium articles, webinars, resources to make the best decisions for career, course, exams, scholarships, study abroad and much more with. The greatest example of a galvanic cell that turns chemical energy into electrical energy is a Daniell cell. The Daniell cell is made up of two electrodes made of different metals, Zn and Cu, which are in contact with a solution of their respective ions, zinc sulphate and copper sulphate. Register Now. A conventional galvanic cell, it is meant to generate an electric current by using the spontaneous redox reaction between zinc and cupric ions. A copper vessel makes up this cell.
Daniell cell class 12
How does a Cell in a T. V remote make it work or how a Battery of Mobile Phone Charges when connected to its charger? All such questions are answered in the branch of Science known as Electrochemistry. Electrochemistry is the study of producing Electricity through Chemical reactions and also the use of Electricity to carry out non-spontaneous Chemical reactions.
Mini skirt xxx
Studying in a group benefits you in maintaining consistency and focus. JEE Application Process. Close suggestions Search Search. Write the overall reaction taking place in Daniel Cell. Daniell cell has the emf value 1. The porous pot cell consists of a central zinc anode dipped into a porous earthenware pot containing a zinc sulfate solution. The salt bridge lets electrons flow. This variant, called a gravity cell, consists of a glass jar in which a copper cathode sat on the bottom and a zinc anode is suspended beneath the rim in the zinc sulfate layer. The copper vessel was filled with sulfuric acid solution saturated with copper sulfate to above the level of the perforated disc. Download Important Formulas pdf.
An electrochemical cell known as a Daniell cell converts chemical energy into electrical energy. To generate electricity, the cell engages in a variety of chemical reactions. The zinc and copper electrodes that make up the Daniell cell are in use as the anode and cathode , respectively.
He was searching for a way to eliminate the hydrogen bubble problem found in the voltaic pile , and his solution was to use a second electrolyte to consume the hydrogen produced by the first. Thus, the following are the two main conditions of reversibility:. At the anode, oxidation takes place and solid zinc converts into zinc ions. While electrolytic cells involve non-spontaneous reactions and therefore need an external electron source, such as a DC battery or an AC power source, galvanic cells get their energy from spontaneous redox reactions. Click Here Know More. JEE Marking Scheme. Electrons flow from anode to cathode in the external circuit. No,a Daniell cell is not rechargeable. Current flows from the voltaic cell into the external source if the emf of the voltaic cell is greater than that of the external source. Experiment In the Daniell cell, copper and zinc electrodes are immersed in a solution of copper II sulfate and zinc sulfate, respectively. Personal Growth Documents. Wikimedia Commons has media related to Daniell cell. Also browse for more study materials on Chemistry here. These processes result in the accumulation of solid copper at the cathode and the corrosion of the zinc electrode into the solution as zinc cations. Similarly, the half-cell occurs is called reduction half-cell and the reaction taking place in it is called reduction half-cell reaction.

0 thoughts on “Daniell cell class 12”