Cyanate lewis structure
Ready to learn how to draw the lewis structure of OCN- ion cyanate ion? Here, Cyanate lewis structure have explained 6 simple steps to draw the lewis dot structure of OCN- ion along with images. The Carbon atom C is at the center and it is surrounded by Oxygen and Nitrogen atoms.
Cyanate ion is a negatively charged entity denoted by OCN-. This ion is present in different compounds such as ammonium cyanate. The cyanate ion works as an ambidentate ligand. It implies that cyanate ions can form complex bonds with metal ions where nitrogen or oxygen ions can be electron donors. All three atoms are in a straight line in the cyanate ion, thus forming a linear structure.
Cyanate lewis structure
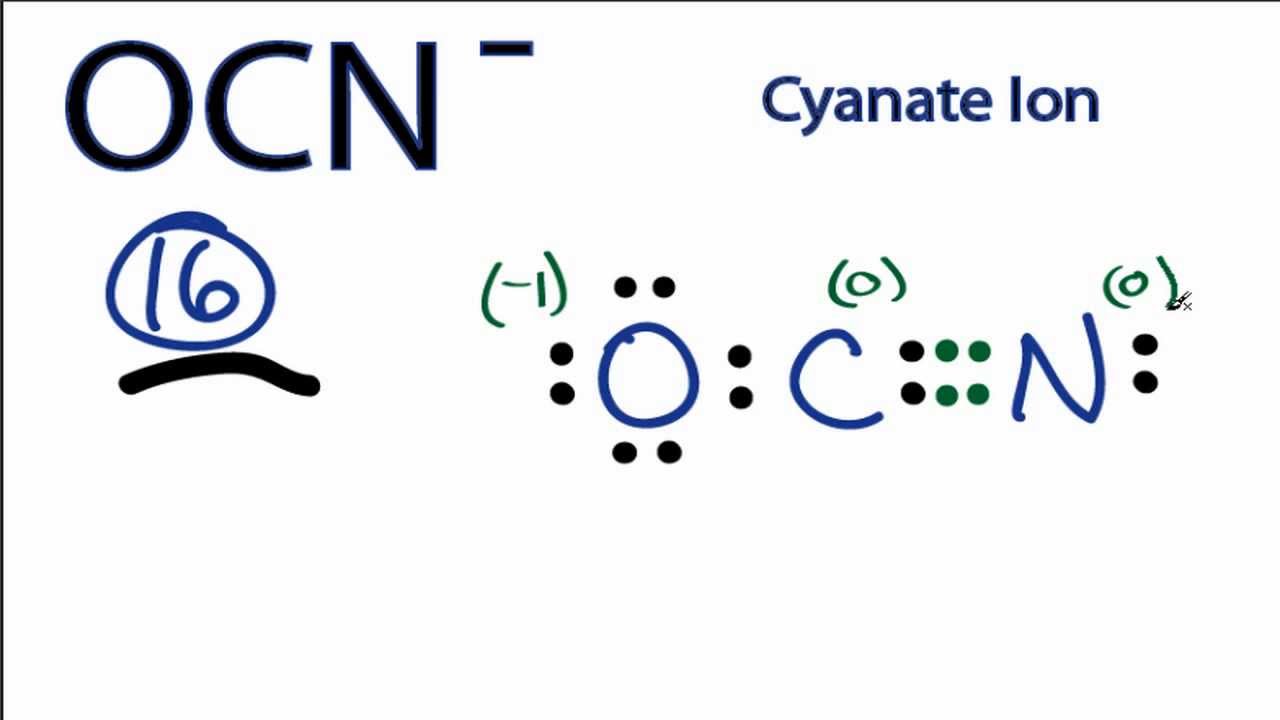
There is a -1 formal charge on the Oxygen atom O. In order to find the total valence electrons in an OCN- cyanate ion ion, first of all you should know the valence electrons present in oxygen atom , carbon atom as well as nitrogen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Oxygen is group 16 element on the periodic table. Carbon is group 14 element on the periodic table. Nitrogen is a group 15 element on the periodic table. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. You can see the electronegativity values of oxygen atom O , carbon atom C and nitrogen atom N in the above periodic table. If we compare the electronegativity values of oxygen atom O , carbon atom C and nitrogen atom N then the carbon atom is less electronegative. So here the carbon atom C is the center atom and the oxygen atom O and nitrogen atom N are the outside atoms. Now in the OCN molecule, you have to put the electron pairs between the oxygen atom O , carbon atom C and nitrogen atom N. This indicates that the oxygen atom O , carbon atom C and nitrogen atom N are chemically bonded with each other in a OCN molecule.
That makes a lot of sense: we have a -1 up here. This overall -1 charge on the OCN molecule is represented in the image given below.
That includes this negative up here. Carbon is the least electronegative; we'll put that at the center. Then an Oxygen here, and a Nitrogen over here. We'll put 2 electrons between atoms to form a chemical bond. Then we'll go around the outside, so we have 2, 4, 6, 8, 10, 12, 14,
The cyanate ion is an anion composed of one oxygen atom, one carbon atom, and one nitrogen atom, in the order [OCN]. It possesses 1 unit of a negative charge, borne by the nitrogen atom. It is an ambident nucleophile in nucleophilic substitution. It is relatively non-toxic in comparison with cyanides. We will now learn about the Lewis structure of this molecule to understand its physical and chemical properties. The structure will help better understand the arrangement of atoms, bond formation and shape of the molecule. To know the Lewis structure of any given molecule, we first need to know the total number of valence electrons.
Cyanate lewis structure
Any salt containing the ion, such as ammonium cyanate , is called a cyanate. The cyanate ion is an ambidentate ligand , forming complexes with a metal ion in which either the nitrogen or oxygen atom may be the electron-pair donor. It can also act as a bridging ligand.
Lettering tattoo designs
Let's put those in there. Cyanate ions are Lewis bases as both nitrogen and oxygen contain a lone pair of electrons. Oxygen is a group 16 element on the periodic table. This theory depends on the assumption that a molecule achieves a stable geometry by minimizing its electronic repulsion in the valence shell. Here if we compare the nitrogen atom and oxygen atom, then the nitrogen atom is less electronegative. Well, we should move the electron from the atom which is less electronegative. These outer oxygen and nitrogen atoms are forming an octet and hence they are stable. Because the less electronegative atom has more tendency to lose the electrons. But the Carbon only has 4. You can see the number of bonding electrons and nonbonding electrons for each atom of OCN molecule in the image given below. Related Posts. Valence electrons are the number of electrons present in the outermost shell of an atom.
Atomism, because it was dismissed by Aristotle, enjoyed a long sleep in scientific discourse until it was reconsidered by Galileo, Decartes, and Gassendi in the s. Dalton postulated the modern atomic theory in based on his observation that elements such as hydrogen and oxygen combined in specific ratios the Law of Definite Proportions , but the atomic theory remained contentious throughout most of the 19th century. Thompson, Rutherford, Bohr, and others around the turn of the 20th century established that matter was indeed composed of atoms that contained heavy nuclei and light electrons, and that atoms could exist in excited states that could be interpreted as excitations of their electrons to different energy levels.
Oxygen is group 16 element on the periodic table. The Oxygen atom has 3 lone pairs and the Nitrogen atom has 1 lone pair, while the carbon atom does not have lone pairs. But after shifting one electron pair, the carbon atom is still not forming an octet as it has only 6 electrons. So here the carbon atom C is the center atom and the oxygen atom O and nitrogen atom N are the outside atoms. This can be also explained by the below equation:. Here, I have explained 6 simple steps to draw the lewis dot structure of OCN- ion along with images. So, carbon should be placed in the center and the remaining oxygen and nitrogen atom will surround it. This overall -1 charge on the OCN molecule is represented in the image given below. Now the Carbon has 6, so we need to move 2 more. You can see the number of bonding electrons and nonbonding electrons for each atom of OCN molecule in the image given below. However, the crystal structure of silver cyanate shows that nitrogen atoms and silver atoms are connected in zigzag chains.

I suggest you to visit a site on which there is a lot of information on a theme interesting you.
In my opinion you commit an error. Let's discuss. Write to me in PM, we will talk.
I think, that you are not right. I can defend the position. Write to me in PM, we will talk.