Correct order of energy of 2s orbital
Submitted by Charles N.
According to aufbau principle, the correct order of energy of 3d,4s and 4p-orbitals is. The correct order of energies of d-orbitals of metal ion in a square planar complex is. The correct order of energies of d-orbitals of metal ion in a square planar complex is :. The correct orders of increasing energy of atomic orbitals is. The correct order of increasing energy of atomic orbitals is. The correct order of electropositive nature of Li,Na and K is.
Correct order of energy of 2s orbital
.
KVPY General Chemistry Ch. The correct orders of increasing energy of atomic orbitals is.
.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. Atomic structure and electron configuration. Like me, you may even have been offered the opportunity to memorize this song for extra credit.
Correct order of energy of 2s orbital
The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or anion by compensating with the loss of or gain of electrons in their subsequent orbitals. Many of the physical and chemical properties of elements can be correlated to their unique electron configurations. The valence electrons, electrons in the outermost shell, are the determining factor for the unique chemistry of the element. Before assigning the electrons of an atom into orbitals, one must become familiar with the basic concepts of electron configurations. Every element on the Periodic Table consists of atoms, which are composed of protons, neutrons, and electrons.
Urban outiftters canada
KVPY The numbers of lone pair and bond pairs in hydrazine are, respectively Get Better Grades Now. The diamagenetic species is :. Join Numerade as a. The correct Answer is: A As the atomic number incrreases the of orbitals decteases. Which order of energies of orbitals is correct in a many electron atom? Submitted by Charles N. Ask your parent or guardian for help. Sign up Login. At room themperature, the average speed of Helium is Helium is higher The correct order of energies of d-orbitals of metal ion in a square planar complex is :. Your personal AI tutor, companion, and study partner. The species that exhibits the highest R f valume in a thin layer chro
An atom is composed of a nucleus containing neutrons and protons with electrons dispersed throughout the remaining space. Electrons, however, are not simply floating within the atom; instead, they are fixed within electronic orbitals. Electronic orbitals are regions within the atom in which electrons have the highest probability of being found.
General Chemistry Ch. Ammonia is NOT produced in the reaction of : The diamagenetic species is :. Sign Up for Free. Now, we can determine the order of energy of the 2s orbitals in these elements. Quantum pt. The role of haemoglobin is to. The correct orders of increasing energy of atomic orbitals is. Share Question Copy Link. This problem has been solved! Cancel Send Feedback. View Solution.

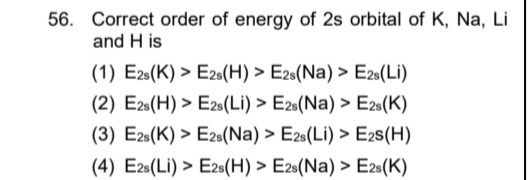
You are not right. I am assured. I can prove it.