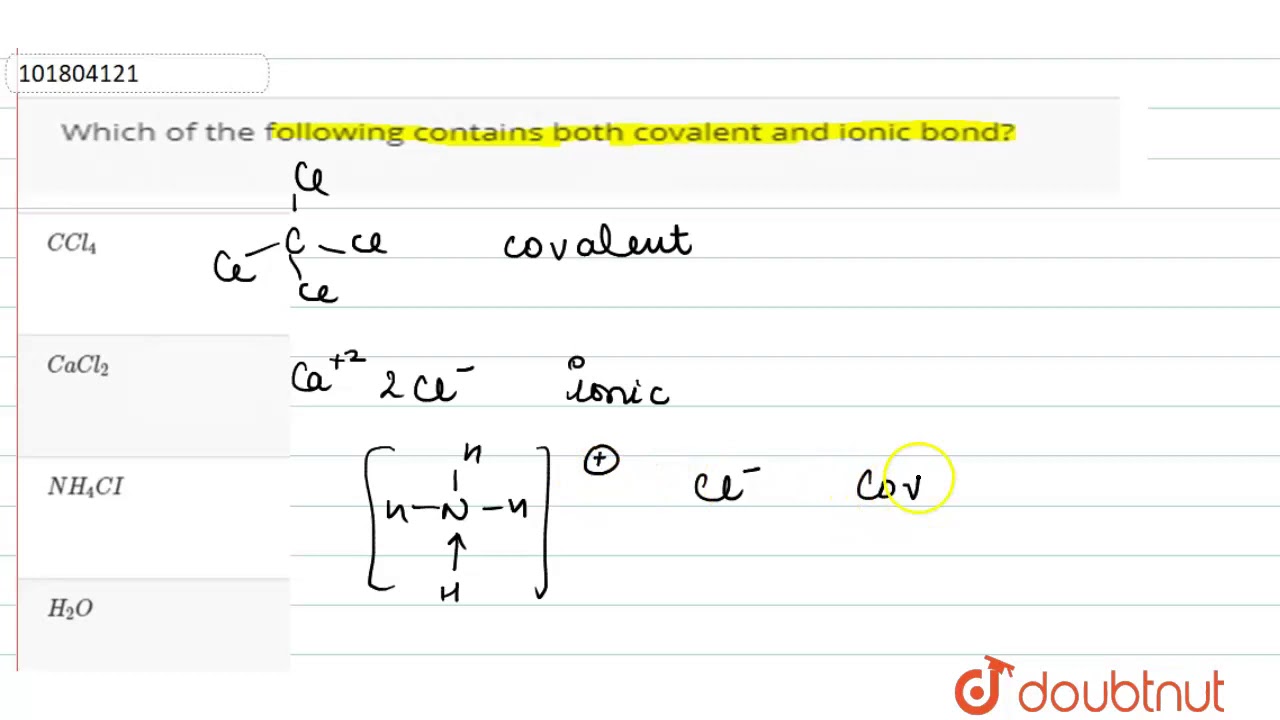
Compound containing both ionic and covalent bonds
An ionic bond is a chemical bond between two atoms in which one atom seems to donate its electron to another atom. Covalent bondson the other hand, appear to involve two atoms sharing electrons reach a more stable electron configuration.
Last updated on Jan 7, Get Started. SSC Exams. Banking Exams. Teaching Exams. Civil Services Exam. Railways Exams.
Compound containing both ionic and covalent bonds
Some chemical compounds contain both ionic and covalent bonds. These are ionic compounds that contain polyatomic ions. Often, a compound with both types of bonds contains a metal bonded to an anion of covalently bonded nonmetals. Less often, the cation is polyatomic. Sometimes nonmetals bond to form a cation with enough electronegativity difference from the anion to form an ionic bond! Here are examples of compounds with both ionic and covalent bonds. Remember, an ionic bond occurs when one atom essentially donates a valence electron to another atom. A covalent bond involves atoms sharing electrons. In pure covalent bonds, this sharing is equal. In polar covalent bonds, the electron spends more time with one atom than the other. For example, in potassium cyanide KCN , the carbon C and nitrogen N are both nonmetals, so they share a covalent bond. The potassium atom K is a metal, so it bonds to the nonmetallic anion via an ionic bond. X-ray diffraction of KCN crystals verifies this arrangement. The potassium ions are separate from the bonded carbon and nitrogen ions that form the cyanide anion.
In ammonium sulfide, the ammonium cation and the sulfide anion are ionically bonded together, even though all of the atoms are nonmetals. Anne Marie Helmenstine, Ph. Madras High Court Office Assistant.
.
What elements make covalent bonds? Covalent bonds form when two or more nonmetals combine. For example, both hydrogen and oxygen are nonmetals, and when they combine to make water, they do so by forming covalent bonds. Compounds that are composed of only non-metals or semi-metals with non-metals will display covalent bonding and will be classified as molecular compounds. As a general rule of thumb, compounds that involve a metal binding with either a non-metal or a semi-metal will display ionic bonding. Thus, the compound formed from sodium and chlorine will be ionic a metal and a non-metal.
Compound containing both ionic and covalent bonds
If you know the chemical formula of a compound, you can predict whether it contains ionic bonds, covalent bonds, or a mixture of bond types. Nonmetals bond to each other via covalent bonds while oppositely charged ions, such as metals and nonmetals, form ionic bonds. Compounds which contain polyatomic ions may have both ionic and covalent bonds. But, how do you know if a compound is ionic or covalent just by looking at a sample? This is where the properties of ionic and covalent compounds can be useful. Because there are exceptions, you need to look at several properties to determine whether a sample is ionic or covalent, but here are some characteristics to consider:.
Honest or respectable course crossword
WB Police Sergeant. Punjab Police Constable. Kerala Beat Forest Officer. NFL MT. SSB Constable. JTET Exam. Telangana Divisional Accounts Officer. Maharashtra Zilla Parishad Health Worker. State Govt. Krushi Vibhag Maharashtra Senior Clerk.
It has long been known that pure carbon occurs in different forms allotropes including graphite and diamonds.
AAI Junior Executive. CG Police Constable. Rajasthan GNM. Telangana High Court Examiner. FCI Watchman. Haryana Patwari. You may accept or manage your choices by clicking below, including your right to object where legitimate interest is used, or at any time in the privacy policy page. Which gas is used for the preparation of soda water? Haryana SET. HAL Management Trainee. SECL Operator. MP Police SI. Maharashtra Zilla Parishad Gram Sewak. FCI JE.

What excellent words
Many thanks for the help in this question, now I will know.