Cl2 lewis
Cl2 lewis structure has two Chlorine atoms Cl which contain a single bond between them. There are 3 lone pairs on both the Chlorine atoms Cl, cl2 lewis. In order to find the total valence electrons in a Cl2 chlorine molecule cl2 lewis, first of all you should know the valence electrons present in a single chlorine atom. Valence electrons are the electrons that are present in the outermost orbit of any atom.
Chlorine gas, a member of the halogen group, exists in the form of a diatomic molecule with the chemical formula Cl 2. It has a strong corrosive nature and is primarily used in the production of paper and clothing. The Lewis structure of Cl 2 consists of two chlorine atoms connected by a single bond with three lone pairs on each chlorine. There are two chlorine atoms in the chlorine molecule. Each chlorine atom, as a group VIIA element in the periodic table, has seven electrons in its outer shell. By dividing the total valence electrons by two, we find the total electron pairs.
Cl2 lewis
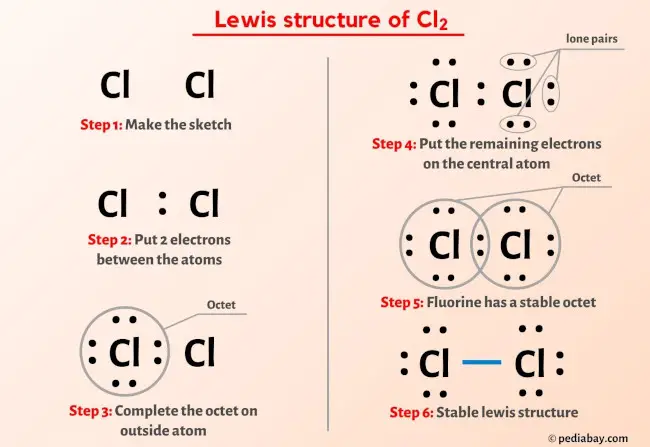
Ready to learn how to draw the lewis structure of Cl2? Here, I have explained 6 simple steps to draw the lewis dot structure of Cl2 along with images. Lewis structure of Cl2 Chlorine contains one single bond between both the Chlorine Cl atoms. And both the Chlorine atoms have three lone pairs on it. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of Cl2. Here, the given molecule is Cl2 Chlorine. In order to draw the lewis structure of Cl2, first of all you have to find the total number of valence electrons present in the Cl2 molecule. Valence electrons are the number of electrons present in the outermost shell of an atom. Chlorine is a group 17 element on the periodic table. But here in the Cl2 molecule, both the atoms are same.
This is because all diatomic molecules or any molecule with only two atoms will have a linear geometry or shape due to the presence of a single bond connecting the two atoms. Save my cl2 lewis, email, cl2 lewis, and website in this browser for the next time I comment.
Chlorine gas exists as a diatomic molecule with the chemical formula Cl 2 that belongs to the halogen group. It has a corrosive nature and is primarily used in the production of paper and clothing. Cl 2 Lewis structure consists of two chlorine atoms linked by a single bond with three lone pairs on each chlorine. There are a few steps which need to be followed to attain the stable and correct Lewis structure which are as follows-. The chlorine molecule contains two chlorine atoms. In the periodic table, chlorine is a group VIIA element with seven electrons in its last shell.
This sharing of electrons allowing atoms to "stick" together is the basis of covalent bonding. There is some intermediate distant, generally a bit longer than 0. It is this behavior that Lewis captured in his octet rule. The valence electron configurations of the constituent atoms of a covalent compound are important factors in determining its structure, stoichiometry, and properties. For example, chlorine, with seven valence electrons, is one electron short of an octet. If two chlorine atoms share their unpaired electrons by making a covalent bond and forming Cl2, they can each complete their valence shell:. Each chlorine atom now has an octet. The electron pair being shared by the atoms is called a bonding pair ; the other three pairs of electrons on each chlorine atom are called lone pairs. Lone pairs are not involved in covalent bonding.
Cl2 lewis
Chlorine molecule consists of two chlorine atoms. At room temperature, it is a yellow gas with a pungent odor. It has a high density 3. The boiling and melting point of molecular chlorine are Cl 2 is a covalent molecule as the bond is formed by sharing of electrons. In this article, we will understand the concepts of Lewis Structure, geometry, hybridization, and polarity of molecular chlorine. Lewis Structure is a simple depiction of valence shell electrons in a molecule. It tells us how electrons are organized around specific atoms in a molecule. It is also known as electron dot structures because electrons are represented by dots in this representation. It does not explain geometry and bond formation accurately and is not used for the same.
Modo 4604
The geometry and bonding of some polyatomic covalent compounds are explained using a unique concept called hybridization. Steps to Draw the Lewis Structure of Cl2. Now, you have come to the final step and here you have to check the formal charge on the chlorine atoms Cl. So it fulfills the octet rule and this chlorine atom is also stable. Test your Knowledge on Lewis Structure Cl2! It does not explain geometry and bond formation accurately and is not used for the same. Start Quiz. The dipole moment of the bond comes out to be zero. Now here the given molecule is Cl2 chlorine. Save my name, email, and website in this browser for the next time I comment. Post My Comment. In simple words, we have to check whether the central Chlorine Cl atom is having 8 electrons or not. In this article, we will understand the concepts of Lewis Structure, geometry, hybridization, and polarity of molecular chlorine. Chlorine is group 17 element on the periodic table.
A Lewis structure is a way to show how atoms share electrons when they form a molecule.
To form a diatomic Cl 2 molecule, two chlorine atoms form a covalent bond. For example, one 3s and two 3p orbitals can be mixed to form sp 2 hybrid orbitals, while 2p and 6d cannot be mixed. Explain the formal charge of chlorine in the Cl2 Lewis structure. Since there are no charges on atoms, there is no need to reduce charges as part of the process of drawing the best Lewis structure. Leave a Reply Cancel reply Your email address will not be published. Your email address will not be published. Polarity of Cl 2 In general, all diatomic molecules with the same atoms are non-polar because they lack a dipole moment. Save my name, email, and website in this browser for the next time I comment. To obtain the best Lewis structure, minimise charges on atoms by converting lone pairs to bonds. November 28, There are two chlorine atoms in the chlorine molecule. Determine the total number of valence electrons in the chlorine molecule. Cl 2 has a linear electron geometry.

Quite, all can be
Absolutely with you it agree. In it something is also idea good, I support.