Chloride lewis dot structure
The number of electrons in the outermost shell of an atom determines its chemical characteristics, chloride lewis dot structure. We visualize valence electrons using Lewis dot structures to locate stable electron configurations. In order to attain stability in any atom like noble gasesthe majority of atoms often lose or gain electrons. In this article, we will learn how to draw the Lewis dot structure of Sodium Chloride stepwise.
In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. In this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely Lewis symbols and Lewis structures. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons:. Figure 1. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium: Likewise, they can be used to show the formation of anions from atoms, as shown below for chlorine and sulfur: Figure 2 demonstrates the use of Lewis symbols to show the transfer of electrons during the formation of ionic compounds.
Chloride lewis dot structure
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot symbol or electron dot diagram or a Lewis diagram or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. It does not matter what order the positions are used. Figure 1. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium: Likewise, they can be used to show the formation of anions from atoms, as shown below for chlorine and sulfur: Figure 2 demonstrates the use of Lewis symbols to show the transfer of electrons during the formation of ionic compounds. Figure 2. Cations are formed when atoms lose electrons, represented by fewer Lewis dots, whereas anions are formed by atoms gaining electrons. The total number of electrons does not change. The valence electron configuration for aluminum is 3 s 2 3 p 1.
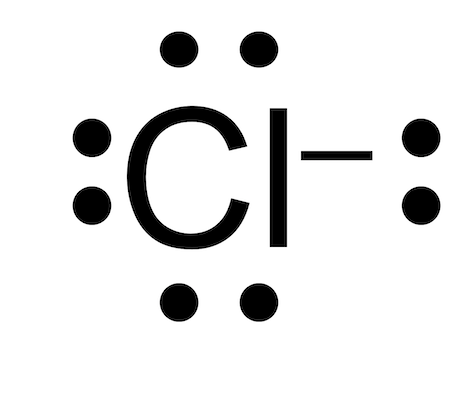
Lewis electron dot diagrams for ions have fewer for cations or more for anions dots than the corresponding atom. See these examples:. Richard Smalley —
.
When two atoms approach each other, they have the potential to bond or connect. If a metal and a nonmetal interact, then an ionic bond will result. These types of bonds involve the metal donating its valence electron s to a nonmetal, forming an ionic compound. As the transfer of electrons occurs, both atoms will achieve a more stabile confirmation. The end result will be a less reactive compound.
Chloride lewis dot structure
In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. In this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely Lewis symbols and Lewis structures. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons:. Figure 7. Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium:. Likewise, they can be used to show the formation of anions from atoms, as shown here for chlorine and sulfur:. We also use Lewis symbols to indicate the formation of covalent bonds, which are shown in Lewis structures , drawings that describe the bonding in molecules and polyatomic ions. For example, when two chlorine atoms form a chlorine molecule, they share one pair of electrons:.
Cernovich twitter
Molecular Structure of Compounds. We visualize valence electrons using Lewis dot structures to locate stable electron configurations. Richard Smalley — Since this is a diatomic molecule with only two atoms, selecting the central atom with the lowest electronegativity is not required. If t is the total number of electrons and n is the number of single bonds, t — 2n electrons remain to be placed. What are the Lewis structures of these two molecules? A Lewis structure is a type of diagram used to represent the electron configuration of a molecule or ion. In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. Remember that H is never a central atom:. Both methanol and ethanol produce CO 2 and H 2 O when they burn. Each atom in the molecule is assigned a pair of lone electrons. Distribute the remaining electrons as lone pairs on the terminal atoms except hydrogen to complete their valence shells with an octet of electrons. Licenses and Attributions. The number of dots around an atom represents the number of electrons that are available for bonding with other atoms in the molecule.
Keywords cation, anion, Madelung constant, enthalpy, valence electron, Gilbert Lewis, ionization, isoelectronic, metal, nonmetal, ionic bond, electron transfer, electron sharing, covalent bond, percent ionic character, homonuclear bond, heteronuclear bond, triple bond, dative bond, s and p orbitals, Lewis structures, Linus Pauling, hybrid orbital, crystallization energy, bond energy, charge displacement, dipole moment, polar covalency, electronegativity, polar bond, polar molecule.
The total number of electrons does not change. This can be done as-. The most electronegative atoms are generally assigned to the lone pairs first. The reactivity of the compound is also consistent with an electron deficient boron. The octet rule only takes into account the s and p electrons; it ignores the d and f electrons. Lone pairs, unpaired electrons, and single, double, or triple bonds are used to indicate where the valence electrons are located around each atom in a Lewis structure. This is to ensure that the octet rule may be followed, which only allows for a maximum of eight valence electrons. Write the formula of each compound using the chemical symbols of each element: Write the Lewis structure for the diatomic molecule P 2 , an unstable form of phosphorus found in high-temperature phosphorus vapor. It is possible to draw a structure with a double bond between a boron atom and a fluorine atom in BF 3 , satisfying the octet rule, but experimental evidence indicates the bond lengths are closer to that expected for B—F single bonds. In this case, we can condense the last few steps, since not all of them apply. To draw the Lewis structure for an odd-electron molecule like NO, we follow the same six steps we would for other molecules, but with a few minor changes:.

It is a pity, that now I can not express - there is no free time. I will be released - I will necessarily express the opinion.
Yes, the answer almost same, as well as at me.
Understand me?