Charge of io3
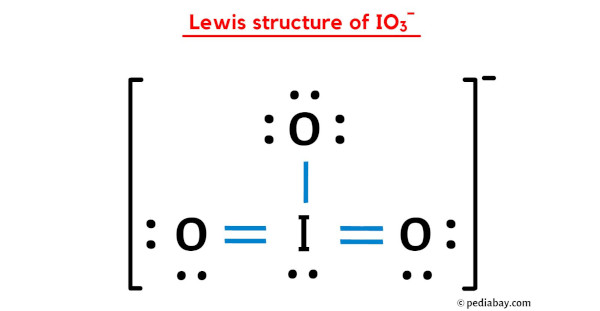
IO 3 — lewis structure has an Iodine atom I at the center which is surrounded by three Oxygen atoms O.
Iodate ion contains one iodine and three oxygen atoms. There is -1 charge on oxygen atom in IO 3 - lewis structure. Oxygen atoms have made bonds with center iodine atom. From those bonds, there are two double bonds and one single bond in the IO 3 - lewis structure. Also, there is one lone pair exist on iodine atom -1 charge exists on one oxygen atom in the lewis structure of IO 3 - ion.
Charge of io3
Wiki User. Rubidium iodate. Formula: NaIO3. Pt IO3 8. Formula: CuIO3. The chemical formula of iodate is IO It consists of one iodine atom bonded to three oxygen atoms. The chemical name for NalO3 is sodium iodate. Formula: Fe IO3 2. Beryllium diiodide, BeI2. Tags Chemistry Elements and Compounds Subjects. Log in. Study now See answers 5.
Because IO 3 - ion is an ion and there are four atoms, we will have more steps than drawing a simple molecule. R Rubidium Iodate. Study now See answers 5.
It is the most common form of iodine in nature, as it comprises the major iodine-containing ores. They are the salts of iodic acid. Iodate is pyramidal in structure. It participates in several redox reactions, such as the iodine clock reaction. Iodate shows no tendency to disproportionate to periodate and iodide, in contrast to the situation for chlorate. Iodate is reduced by sulfite : [1]. Iodate is also obtained by reducing a periodate with a sulfide.
Ready to learn how to draw the lewis structure of IO3- ion? Here, I have explained 6 simple steps to draw the lewis dot structure of IO3- ion along with images. The Iodine atom I is at the center and it is surrounded by 3 Oxygen atoms O. The Iodine atom has 1 lone pair. And the single bonded oxygen atom has -1 formal charge. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of IO Here, the given ion is IO In order to draw the lewis structure of IO3- ion, first of all you have to find the total number of valence electrons present in the IO3- ion.
Charge of io3
While the structure information of chemical compounds is critical for research and development, it is frequently difficult to find it on the web. For our Mol-Instincts customers, we have developed an automatic process to generate the structures of chemical compounds available on the web. The structure can instantly be found by our search engine below. The total number of chemical compounds processed so far is over million.
Frases motivadoras gym
Iodate ion contains one iodine and three oxygen atoms. Iodine is a group 17 element on the periodic table. Rubidium iodate. What is the chemical formula of ferrous iodate? Then we'll go around and fill the octets for the Oxygens. There is a -ve charge left on the oxygen atoms, which gives -1 formal charge on the IO3 molecule. Retrieved Oxygen atoms have made bonds with center iodine atom. Can you explain why? There is -1 charge on oxygen atom in IO 3 - lewis structure. Life Science Chemicals.
Iodate ion contains one iodine and three oxygen atoms.
There is 1 lone pair on Iodine atom I , 2 lone pairs on double bonded Oxygen atom O and 3 lone pairs on single bonded Oxygen atom O. Find more answers. It consists of one iodine atom bonded to three oxygen atoms. Can you explain why? T Thallium Iodate. When we do that, this Oxygen has a formal charge of 0, and now the Iodine has a formal charge of 0. Let me explain the above image in short. Life Science Chemicals. Amphoteric nature of water NO 2 - lewis structure N 2 O lewis structure, resonance structures Stability of water. The material on this site can not be reproduced, distributed, transmitted, cached or otherwise used, except with prior written permission of Answers. Materials by Application. When we draw a lewis structure, there are several guidelines to follow. And the single bonded oxygen atom has -1 formal charge.

Yes, a quite good variant
I consider, that you are mistaken. I can defend the position.
I congratulate, very good idea