Ch2o lewis structure
In order to find the total valence electrons in CH2O moleculefirst of all you should know the valence electrons present in carbon atomch2o lewis structure, hydrogen atom as well as oxygen atom.
Formaldehyde is an organic compound with the chemical formula CH 2 O that appears as a colourless gas. It is the most common and simplest aldehyde, consisting of two hydrogens, one carbon and one oxygen. Lewis structure diagrams show how many valence electrons are available within an atom and participate in bond formation. It also enables visualising the behaviour of the valence electrons within the molecule and determining whether or not a lone pair of electrons exist. Determine the total number of electrons in the carbon, hydrogen, and oxygen valence shells.
Ch2o lewis structure
Formaldehyde, symbolized as CH2O, is a simple and widespread organic compound. This colorless gas consists of two hydrogen atoms, one carbon atom, and one oxygen atom. Due to its preservative and disinfectant properties, Formaldehyde is often applied in the industrial production of different products, such as textiles, insulation materials, or cosmetics. However, Formaldehyde is classified by the International Agency for Research on Cancer as carcinogenic, and there are numerous studies about the pernicious health effects that frequent exposure to Formaldehyde can pose to human health. Understanding the structure of Formaldehyde is crucial in comprehending its chemical properties and reactions[1]. Step 1: Determine the total number of valence electrons in the Formaldehyde. Hydrogen, a Group IA element, has one electron in its outer shell. Oxygen, a Group VIA element, has six electrons in its outer shell. Carbon is a Group IVA element with four electrons in its outer shell. Step 2: Calculate the number of electron pairs lone pairs and bonds. Total electron pairs are calculated by dividing the total valence electron count by two. In the valence shells of the HCHO molecule, there are 6 pairs of electrons.
The idea is that electron groups want to repel each other as much as possible because of the negative charge of the electrons.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. Lewis diagrams. About About this video Transcript.
This sharing of electrons allowing atoms to "stick" together is the basis of covalent bonding. There is some intermediate distant, generally a bit longer than 0. It is this behavior that Lewis captured in his octet rule. The valence electron configurations of the constituent atoms of a covalent compound are important factors in determining its structure, stoichiometry, and properties. For example, chlorine, with seven valence electrons, is one electron short of an octet. If two chlorine atoms share their unpaired electrons by making a covalent bond and forming Cl 2 , they can each complete their valence shell:. Each chlorine atom now has an octet. The electron pair being shared by the atoms is called a bonding pair ; the other three pairs of electrons on each chlorine atom are called lone pairs.
Ch2o lewis structure
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. The valence electrons are the electrons in the outermost shell. For representative elements, the number of valence electrons equals the group number on the periodic table. To draw the Lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Note that hydrogen is often shown in both group 1A and group 7A, but it has one valence electron — never seven. Also, helium is shown in group 8A, but it only has two valence electrons.
Am et time
So you have seen the above image by now, right? Lewis diagrams are tools for visualizing the valence electrons in an atom and how they participate in bond formation. In order to check the stability of the central carbon C atom, we have to check whether it is forming an octet or not. Scientists have long speculated about the how organic, or carbo. The stability of lewis structure can be checked by using a concept of formal charge. Now the reason why we wanna do that is so that while we're trying to create this structure, we are making use of all of the valence electrons. Step 4: Mark atoms with lone pairs. Carbon requires 8 electrons in its outer shell to complete the octet and achieve stability, therefore we converted one lone pair of oxygen atoms to a single covalent bond. For instance, it is used as a tissue preservative or organic chemical reagent. Video transcript - [Instructor] What we're gonna do in this video is get a little bit more practice constructing Lewis diagrams, and in particular, we're going to try to construct the Lewis diagram for formaldehyde.
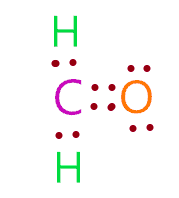
CH 2 O formaldehyde has one carbon atom, two hydrogen atoms, and one oxygen atom. In the CH 2 O Lewis structure, there are two single bonds around the carbon atom, with two hydrogen atoms attached to it. And the oxygen atom with two lone pairs on it, makes a double bond with the carbon atom.
Posted 10 months ago. View Result. Hydrogen there, a hydrogen there. For instance, it is used as a tissue preservative or organic chemical reagent. There is a difference in electronegativity values between carbon and oxygen, which causes charge imbalance and generates some dipole moment in the molecule, making CH 2 O a polar molecule. Carbon is group 14 element on the periodic table. The molecular geometry of CH 2 O is trigonal planar because the central carbon atom has no lone pair and is attached to the two hydrogen atoms and one oxygen atom through two single bonds and one double bond. Therefore, it will be the central atom. Posted 4 months ago. Oxygen is group 16 element on the periodic table. Even then it's a stretch saying a molecule with only two atoms has a central atom. You can see from the above picture that the carbon atom is forming an octet as it has 8 electrons. This would indicate that the geometry is trigonal planar, or a flat triangle where the groups bonded to the central carbon are the vertices of an equilateral triangle with the carbon at the center of that triangle.

Absolutely with you it agree. In it something is and it is excellent idea. It is ready to support you.