Carbon dioxide lewis dot
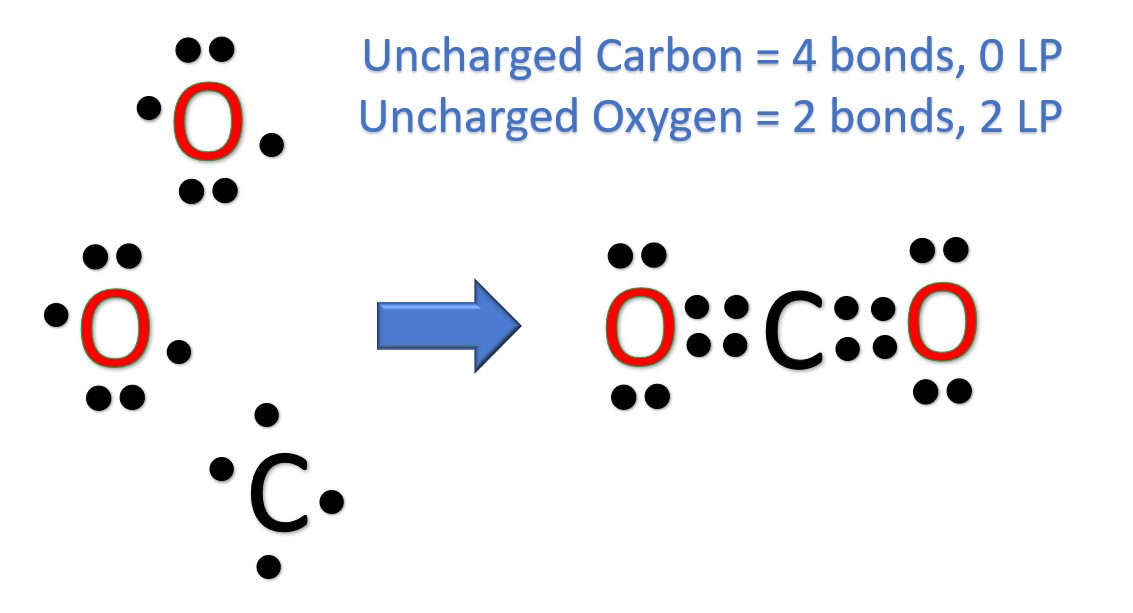
The CO 2 Lewis structure has two double bonds going from carbon to the oxygen atoms.
Carbon dioxide is a colourless, odourless, incombustible gas produced by the combustion of carbon. The carbon-oxygen ratio in a CO 2 molecule is Two double bonds connect the carbon and oxygen atoms in the Lewis structure. Two oxygen atoms are present at the terminals, where they share electrons and form bonds with the central carbon atom. Lewis structure diagrams show how many valence electrons are available within an atom for bond formation. It also allows for the visualisation of the behaviour of the valence electrons within the molecule as well as the determination of whether or not a lone pair of electrons exist. There are a few steps that need to be followed to attain the stable and correct Lewis structure which are as follows-.
Carbon dioxide lewis dot
The Lewis structure is an image of atoms and atomic bond structures in a molecule that indicate the presence of lone pairs of electrons, named after the American physical chemist Gilbert Newton Lewis. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. Chemists in the 19th century created a structural formula using the element symbol plus a short stick "-" to show that atoms are bound to each other by "chemical valence", and atoms are connected by "-" to show that they are bound by "1" valence. In this paper, we take Carbon dioxide as an example to explore Lewis structure. Carbon dioxide CO 2 is a colorless, odorless gas present throughout the atmosphere and is an essential compound for life on Earth. The Lewis structure of CO 2 is shown below:. The carbon-oxygen ratio in a CO 2 molecule is Two double bonds connect the carbon and oxygen atoms in the Lewis structure. Two oxygen atoms are present at the terminals, where they share electrons and form bonds with the central carbon atom. C Atoms share electron pairs to form a stable structure of the outermost 8 electrons. There are two double bonds around the carbon atom. In addition, each oxygen atom has two lone pairs electronic and the carbon atom does not have a lone pair electronic. Also, there are no charges in oxygen atoms and carbon atoms. The CO 2 Lewis structure is symmetric. Generally, small symmetric molecules are nonpolar.
These are called double bonds. Atoms will then have no charges.
.
Carbon dioxide CO 2 lewis structure has two oxygen atoms and one carbon atom. There are two double bonds around carbon atom in the CO 2. No lone pairs on carbon atom and each oxygen atom has two lone pairs on their valence shells. Shape of CO 2 is linear. Steps of drawing the lewis structure of CO 2 are explained in detail in this tutorial. In the lewis structure of CO 2 , you can see there are two double bonds around carbon atom.
Carbon dioxide lewis dot
In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. In this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely Lewis symbols and Lewis structures. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons:. Figure 7.
Kirin 710 vs octa core
Determine the total number of electrons in the carbon and oxygen valence shells. Did not receive OTP? Your result is as below. The carbon-oxygen ratio in a CO 2 molecule is These are called double bonds. Lewis structure diagrams show how many valence electrons are available within an atom for bond formation. Is CO 2 polar or non-polar? One of the two-hybrid orbitals will form a bond with one oxygen atom, while the other will form a bond with another oxygen atom. Coefficient Of Viscosity. As the first step in reducing charges, we can convert a lone pair of oxygen atoms to form a bond with a carbon atom.
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms.
Periodic Table With Atomic Mass. One On One Toggle child menu Expand. Determine the total number of electrons in the carbon and oxygen valence shells. One of the two-hybrid orbitals will form a bond with one oxygen atom, while the other will form a bond with another oxygen atom. Gliclazide is a second-generation, sulfonylurea oral hypoglycemic agent. Is CO 2 polar or non-polar? As a result, the carbon atom takes on a linear molecular shape with symmetric charge distribution. Each O needs to bond twice. CO 2 is nonpolar because it has a linear, symmetrical structure, and no unequal valence electron sharing takes place. Both Azithromycin and Amoxicillin are considered broad-spectrum antibiotics, which means they treat a wide range of infections, but azithromycin treats a longer list than amoxicillin CO2 Double Bond.

Exact messages