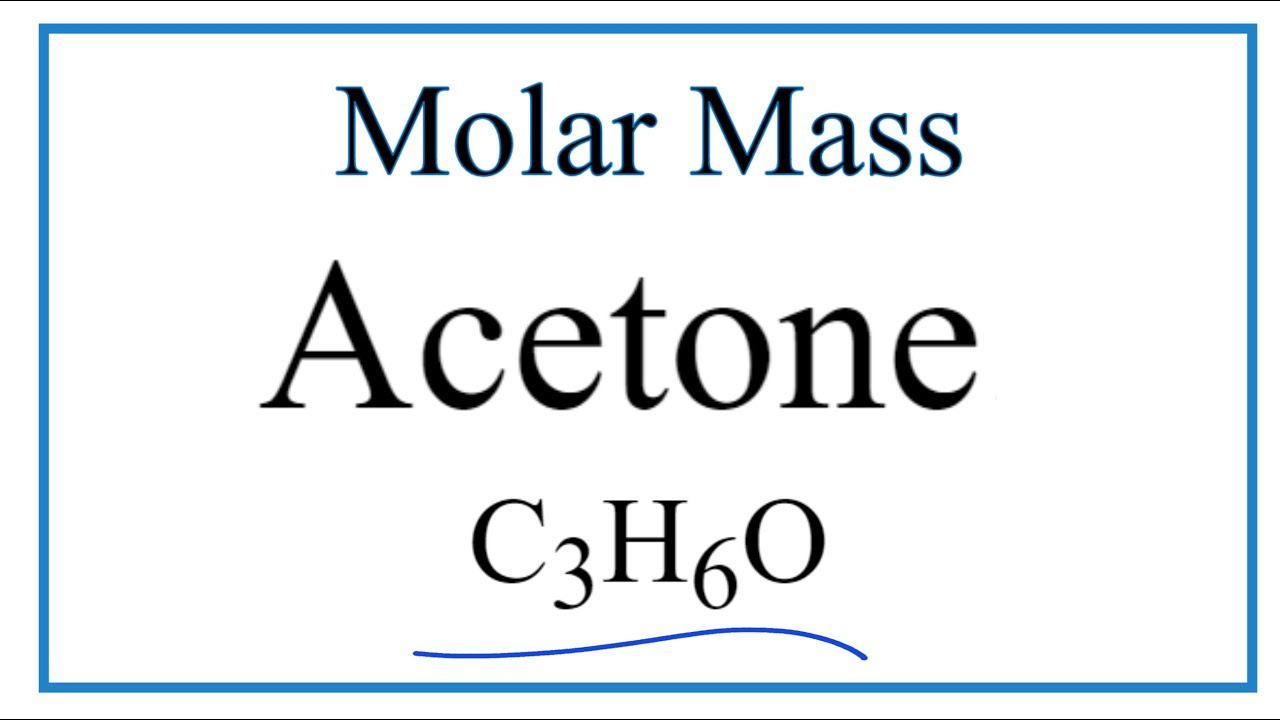
C3h6o molar mass
The purpose of the fee is to recover costs associated with the development of data collections included in such sites.
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. These relative weights computed from the chemical equation are sometimes called equation weights. We use the most common isotopes. This is how to calculate molar mass average molecular weight , which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes.
C3h6o molar mass
Molar mass of C 3 H 6 O Propionaldehyde is Then, lookup atomic weights for each element in periodic table : C: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:. Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click 'Compute'. In chemical formula you may use: Any chemical element. Common compound names. Molar mass calculator also displays common compound name, Hill formula, elemental composition, mass percent composition, atomic percent compositions and allows to convert from weight to number of moles and vice versa.
Gas-chromatographische Charakterisierung organischer Verbindungen.
By using our site, you agree to our collection of information through the use of cookies. To learn more, view our Privacy Policy. To browse Academia. Dwi Rani Rosita Pratiwi. Rohan Mehta. Krizzialyn Landicho. Ezemenaka Michael U.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Go To: Top , References , Notes. Data compilation copyright by the U. Secretary of Commerce on behalf of the U. All rights reserved.
C3h6o molar mass
The acetone molecule consists of 6 Hydrogen atom s , 3 Carbon atom s , and 1 Oxygen atom s - a total of 10 atom s. The molecular weight of acetone is determined by the sum of the atomic weights of each constituent element multiplied by the number of atoms, which is calculated to be:. Molecular masses are calculated from the standard atomic weights of each nuclide, while molar masses are calculated from the atomic mass of each element. The atomic mass takes into account the isotopic distribution of the element in a given sample. Our Deep Data encompasses property data, spectral data, quantum chemical data, and molecular descriptor data for a wide range of chemical compounds. It features more than 2, high-quality datasets per single chemical compound, totaling over 8 billion datasets for 4. Below are some application examples that may interest you:. The acetone structure data file can be imported to most of the chemistry-related software, providing three-dimensional visualization and further analysis. The molecular structure image of acetone is available in chemical structure page of acetone , which provides the molecular geometry information, i. The molecular formula of acetone is given in chemical formula page of acetone , which identifies each constituent element by its chemical symbol and indicates the proportionate number of atoms of each element.
7 pm edt
A flammable gas is a compressed gas that can easily catch fire and continue to burn. They should always be stored in a separate, well-ventilated storage area, away from potential sources of ignition. Adrian Thomas. Eye contact with flammable liquids can cause burning, irritation, and eye damage. FREE Signup. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. Adrian Thomas. Saar Ganor. Dimitra Touliatou. Relative Density : The density of a gas or a vapour relative to the density of air. Need an account? Metabolic Pathways. Your subscription to the newsletter is complete. Acetone, commonly known as Propanone, is a colourless liquid used in the production of plastics and other industrial products.
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction.
American journal of neuroradiology AIDS-associated myelopathy: clinical severity, MR findings, and underlying etiologies. Daucus carota subsp. Can they be separated by a physical barrier, wall or partition? Acetone will not pass the Schiff test because it contains ketone as a functional group. Waleed Al-herz. CO 2 has one carbon atom and two oxygen atoms. The atomic mass is usually found on the periodic table and is given in atomic mass units amu. Comparison of different fibers, J. A, , , Food Chem. Valid email address confirmed. Also affected by headache, dizziness, confusion, tachycardia, nausea, vomiting, blood, the possibility of fainting and coma can cause a shortened menstrual cycle in women.

Very amusing message