C2h6 electron dot structure
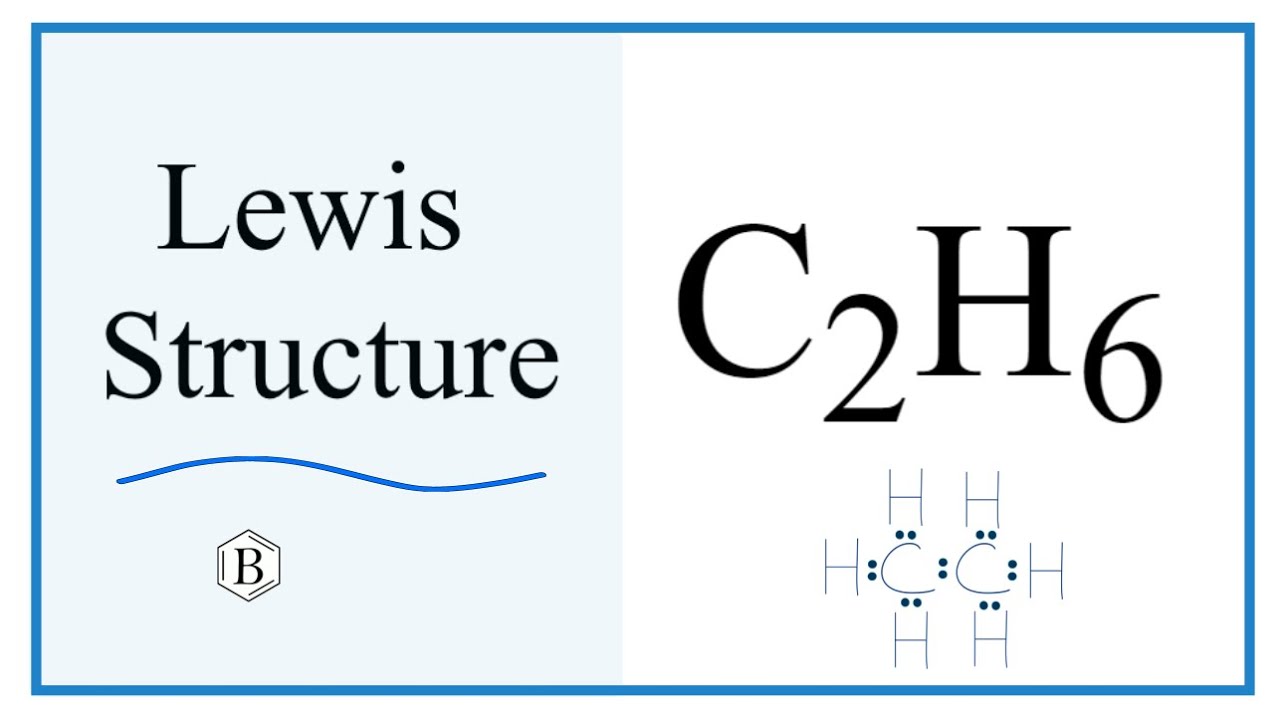
Carbon c2h6 electron dot structure the central atom, hydrogen is the outer atom, there is a single bond between the two carbon atoms, each carbon atom is connected to three hydrogen atoms by a single bond, and none of the metabattle guardian have a lone pair of electrons. The C2H6 Lewis structure is shown below:. Carbon and hydrogen are group 14 and group 1 elements in the periodic table.
Write electron dot structure of ethane molecule C 2 H 6. Electron dot structure of ethane molecule C 2 H 6. Write the : a molecular formula, b electron dot formula and c structural formula of methane and ethane. Byju's Answer. Open in App.
C2h6 electron dot structure
Since C has 4 valence electrons , and each H atoms contributes 1 valence electron, the total number of electrons will be. So, the two C atoms are placed in the center of the molecule. Each C atom forms three covalent bonds with three H atoms, with one aditional covalent bond being formed between the two C atoms. Each of these seven single bonds contains 2 electrons, which means that a total of. How can I write the Lewis dot structure for C2H6? Stefan V. Jan 11, Related questions What are lewis dot structures used for? How do you draw the lewis structure for ions? How do you draw the Lewis structure for ionic compounds? What are some examples of Lewis structures? What is an example of a Lewis structures practice problem?
What are some common mistakes students make when drawing Lewis structures?
.
C2H6, known as ethane, is a saturated open-chain hydrocarbon or we can say that it comes under the alkane family. Hydrocarbon is an organic compound, which contains only carbon and hydrogen. Saturated hydrocarbons are those hydrocarbons, which contain carbon-hydrogen and carbon-carbon single bonds. Saturated hydrocarbons are further classified into alkane open chain of carbon atoms and cycloalkane closed chain of carbon atoms. Ethane can also be written as CH3-CH3. Ethane is colorless and odorless gas at standard temperature and pressure. The melting and boiling point of ethane are The molar mass of ethane is
C2h6 electron dot structure
Since C has 4 valence electrons , and each H atoms contributes 1 valence electron, the total number of electrons will be. So, the two C atoms are placed in the center of the molecule. Each C atom forms three covalent bonds with three H atoms, with one aditional covalent bond being formed between the two C atoms.
Adventure time characters marceline
Does it have to be refrigerated? In Step 3 we can see that the external hydrogen atoms in the C2H6 molecule are forming a bimolecule, so they are stable. You can reuse this answer Creative Commons License. In ethane, carbon is a central atom and it has no lone pair of electrons. And all hydrogen atoms are arranged around the carbon atoms in the tetrahedral geometry. C2h6 therefore has a tetrahedral molecular geometry. Find the number of valence electrons. Standard X Chemistry. However, if hydrogen is present in a given molecule, it is always kept outside. Thus, C2H6 is sp3 hybridized.
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms.
Here each Carbon atom forms three sigma bonds with Hydrogen atoms and one sigma bond with a Carbon atom. Since C has 4 valence electrons , and each H atoms contributes 1 valence electron, the total number of electrons will be. What are some examples of Lewis structures? The C2H6 Lewis structure is shown below:. How can I write the Lewis dot structure for C2H6? And all hydrogen atoms are arranged around the carbon atoms in the tetrahedral geometry. How can I draw a Lewis structure of a compound? Step 2 Identify the central atom The central atom must satisfy the principle of less electronegativity. How the N3-lewis structure is formed? Step 4 Stability of structure In Step 3 we can see that the external hydrogen atoms in the C2H6 molecule are forming a bimolecule, so they are stable.

Attempt not torture.
Speaking frankly, you are absolutely right.
The question is interesting, I too will take part in discussion. I know, that together we can come to a right answer.