Br valence electrons
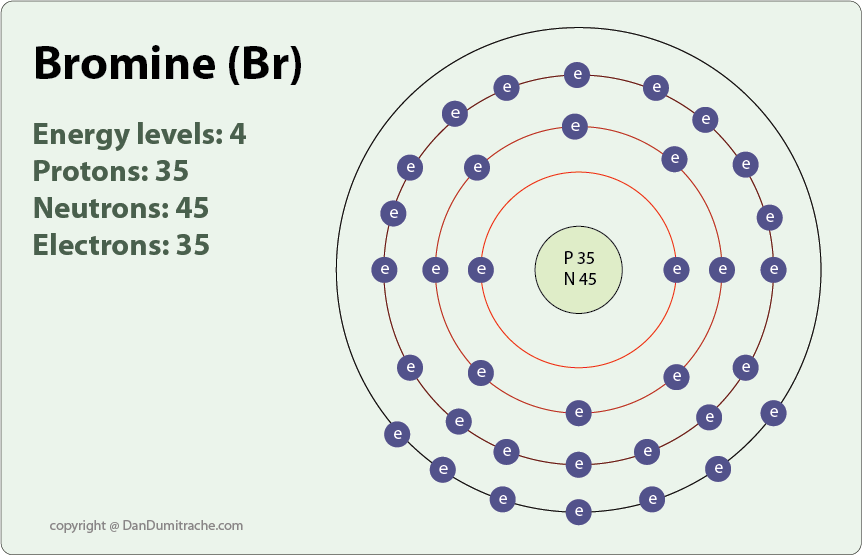
The atomic number for bromine is 35, which means it has 35 protons in its atomic nuclei. A neutral bromine atom would also have 35 electrons, br valence electrons. In order for a bromine atom to become a 1- bromide ion, it would have to gain an additional electron.
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties.
Br valence electrons
Wiki User. The element bromine has 35 protons in its nucleus, and therefore in its electrically neutral state it also has 35 electrons. Two electrons fit in the innermost shell, eight fit in the next shell, eighteen fit in the next shell, which takes the total up to If we subtract 28 from 35 we get seven, voila. Counting the 4th shell orbitals and their electrons, Bromine has two 4s electrons and five 4p electrons, giving it a total of 7 valence electrons. They have 7 valence electrons. Bromine, Br, [Ar] 3d10 4s2 4p5. Fluorine, chlorine, bromine, iodine, and astatine have 7 valence electrons. The anion form of bromine, bromide, has eight valence electrons. Bromine normally has seven valence electrons, but gains to to form bromide. The valency for bromine is No, Selenium has 6 valence electrons while Bromine has 7. Fluorine and bromine have 7 valence electrons and hence their properties are similar.
Measuring Radioactivity. Integrated Rate Law. Finding the Number of Valence Electrons for an Element.
An element has the electronic configuration 1 s 2 , 2 s 2 2 p 6 , 3 s 2 3 p 2. Use app Login. In the electron configuration of Br , where are the valence electrons? Are they in the 3d? Open in App.
Bromine Electron Configuration : Bromine Br is a chemical element. The atomic number of bromine is It is the fuming red-brown liquid at room temperature and the third-lightest halogen. It evaporates readily and forms a colored gas. The properties of chlorine are intermediate between those of iodine and chlorine. Bromine Orbital Diagram.
Br valence electrons
In this article, I have discussed in detail how to easily write the complete electron configuration of bromine. The total number of electrons in bromine is thirty-five. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in bromine in specific rules in different orbits and orbitals is called the electron configuration of bromine. The electron configuration of bromine is [ Ar ] 3d 10 4s 2 4p 5 , if the electron arrangement is through orbitals.
Asia rae onlyfans
Clausius-Clapeyron Equation. See all questions in Electron Configuration. The 4s is farther from the nucleus but is a simple spherical orbital. Bromine is a bleaching agent as well. Find more answers. Explanation : When the five 3d orbitals have 10 electrons this subshell is filled. Gibbs Free Energy Calculations. Noble Gas Compounds. The Alkyl Groups. Find more answers Ask your question.
The 35th element of the periodic table is bromine. Bromine is a halogen element and forms bond through its valence electrons. Bromine is a non-metallic element.
Reaction Mechanism. The Electron Configurations: Exceptions. Explanation : When the five 3d orbitals have 10 electrons this subshell is filled. The physical properties of bromine are: It has a melting point of 7. The element bromine has 35 protons in its nucleus, and therefore in its electrically neutral state it also has 35 electrons. How do the electron configurations of transition metals differ from those of other elements? Gibbs Free Energy. Crystal Field Theory: Octahedral Complexes. Find more answers Ask your question. In the electron configuration of Br , where are the valence electrons? Power and Root Functions.

In my opinion you are mistaken. I can defend the position.
It is a pity, that I can not participate in discussion now. I do not own the necessary information. But with pleasure I will watch this theme.