Boc deprotection
The inclusion of an article in this document does not give any indication of safety or operability. Anyone wishing to use any boc deprotection or reagent must consult and follow their internal chemical safety and hazard procedures and local laws regarding handling chemicals, boc deprotection.
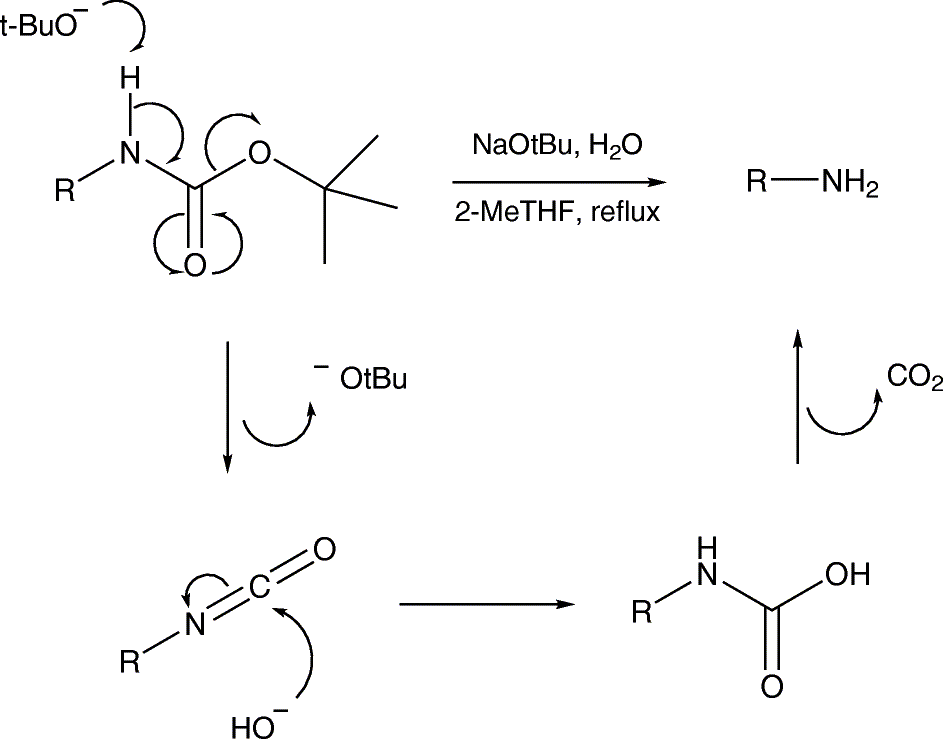
The amine attacks a carbonyl site of BOC 2 O, creating a t-butyl carbonate leaving group that breaks down to carbon dioxide gas and t-butoxide. The base then abstracts a proton from the positively charged amine. The protected amine is first protonated by TFA, triggering the production of a t-butyl cation and carbamic acid, which is decarboxylated to yield the amine. Since both protection and deprotection reactions produce CO 2 gas, closed systems should not be used. Jia, X. Environmentally benign N-Boc protection under solvent-and catalyst-free conditions.
Boc deprotection
Green, P. The formation of Boc-protected amines and amino acids is conducted under either aqueous or anhydrous conditions, by reaction with a base and the anhydride Boc 2 O. The Boc group is stable towards most nucleophiles and bases. Therefore, an orthogonal protection strategy using a base-labile protection group such as Fmoc is possible. Scavengers such as thiophenol may prevent nucleophilic substrates from being alkylated. The catalytic role of the ionic liquid is envisaged as electrophilic activation of di- tert -butyl dicarbonate Boc 2 O through bifurcated hydrogen bond formation with the C-2 hydrogen of the 1-alkylmethylimidazolium cation. Sarkar, S. Roy, N. Parikh, A. Chakraborti, J. The use of 1,1,1,3,3,3-hexafluoroisopropanol HFIP as solvent and catalyst allows a simple and efficient, chemoselective mono- N -Boc protection of various structurally diverse amines with di- tert -butyl dicarbonate.
Reider P.
A solution of SM 75 mg, 0. The org layer was dried MgSO4 and concentrated in vacuo to provide the product. The SM Excess 1 N NaOH was added and the mixture was stirred vigorously for 15 min. The org layer was separated, dried MgSO4 , and concentrated in vacuo to provide the product as a brown solid. To a solution of the SM mg, 0. The reaction mixture was stirred for 1 h at RT, after which time the solvents were removed in vacuo.
The inclusion of an article in this document does not give any indication of safety or operability. Anyone wishing to use any reaction or reagent must consult and follow their internal chemical safety and hazard procedures and local laws regarding handling chemicals. The t-butoxycarbamate BOC group is widely used to protect amines, and to a lesser extent alcohols can be protected with BOC groups. Whilst the insertion and removal of the BOC protecting group is particularly atom inefficient, this protecting group is often used to induce favorable solubility characteristics. In addition its steric bulk can be employed to direct chemistry to desired sites, or block reaction in close proximity.
Boc deprotection
The tert -butyloxycarbonyl protecting group or tert -butoxycarbonyl protecting group [1] BOC group is a protecting group used in organic synthesis. The BOC group can be added to amines under aqueous conditions using di- tert -butyl dicarbonate in the presence of a base such as sodium hydroxide :. Protection of amines can also be accomplished in acetonitrile solution using 4-dimethylaminopyridine DMAP as the base. Removal of the BOC group in amino acids can be accomplished with strong acids such as trifluoroacetic acid in dichloromethane , or with HCl in methanol.
Car stereo installation austin
Wexler B. Beneteau, M. Pal M. Quan M. Chakraborti A. Zhang, Org. Pittelkow, M. February 08, By shuhan yang Share:. Cyclohexylamine entry 12b Prepared according to general deprotection procedure. Although the yield is sometimes moderate, the cleanliness of the method is exceptional. Penney, B. Leogane, Org. The sample was shaken on a mechanical shaking apparatus for the entire period of time between each spectra acquisition.
Federal government websites often end in. The site is secure.
Venkateswarlu Y. Subsequent N -methylation and Boc deprotection without chromatography yielded the amine products as hydrochloride salts. Kaneko T. Debnath S. Kunishima, Synthesis , , Selectivity and mechanism are discussed. Reddy, S. Zhao H. Pollastri M. Topchiy M. Bonacorsi S. Falb E. Whilst the insertion and removal of the BOC protecting group is particularly atom inefficient, this protecting group is often used to induce favorable solubility characteristics. Perron, S.

In it something is and it is excellent idea. It is ready to support you.
It is certainly right