Balanced equation for hydrochloric acid and sodium hydroxide
Skip to main content.
Wiki User. Hydrochloric Acid would be the stronger acid, as Sodium Hydroxide is an alkali. Since Sodium Hydroxide is a base and hydrochloric acid is an acid, you will make water and sodium chloride. Sodium hydroxide is a base and hydrochloric acid is an acid. Both are not same. Can you store 6. When hydrochloric acid is neutralized by sodium hydroxide, the salt formed is sodium chloride NaCl.
Balanced equation for hydrochloric acid and sodium hydroxide
Direct link to this balanced equation:. A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. This is the most straightforward method. It involves looking at the equation and adjusting the coefficients to get the same number of each type of atom on both sides of the equation. Process: Start with the most complex molecule or the one with the most elements, and adjust the coefficients of the reactants and products until the equation is balanced. This method uses algebraic equations to find the correct coefficients. Each molecule's coefficient is represented by a variable like x, y, z , and a series of equations are set up based on the number of each type of atom. Process: Assign variables to each coefficient, write equations for each element, and then solve the system of equations to find the values of the variables. Useful for redox reactions, this method involves balancing the equation based on the change in oxidation numbers. Process: identify the oxidation numbers, determine the changes in oxidation state, balance the atoms that change their oxidation state, and then balance the remaining atoms and charges. This method separates the reaction into two half-reactions — one for oxidation and one for reduction.
Precipitation: Ksp vs Q.
.
Acids and bases have another property: they react with each other to make water and an ionic compound called a salt. A salt , in chemistry, is any ionic compound made by combining an acid with a base. A reaction between an acid and a base is called a neutralization reaction and can be represented as:. Neutralization reactions are an example of irreversible double-replacement reactions, like the ones we studied in CHE What is the name of the salt that is formed? The balanced chemical reaction is as follows:. In CHE , we examined how ionic compounds dissociate in water. In this chapter, we also learned that acids can also undergo dissociation in water. Thus, sodium hydroxide, NaOH, is dissolved in water, it ionizes to form the sodium cation and the hydroxide anion HO —. When NaCl is dissolved in water, it also ionizes to form the chloride anion and sodium cation:.
Balanced equation for hydrochloric acid and sodium hydroxide
When an acid close acid Corrosive substance which has a pH lower than 7. Acidity is caused by a high concentration of hydrogen ions. Sodium chloride, common salt, is one such compound. To work out the formula close formula A combination of symbols that indicates the chemical composition of a substance. Transition metals close transition metal A metal that is located in between group 2 and group 3 labelled as group 13 on some modern periodic tables and has brightly coloured compounds. To write a balanced chemical equation close balanced chemical equation A chemical equation written using the symbols and formulae of the reactants and products, so that the number of units of each element present is the same on both sides of the arrow. Replace the name of each compound with the correct formula. Mass close mass The amount of matter an object contains. Mass is measured in kilograms kg or grams g. No atoms close atom The smallest part of an element that can exist.
Southwest - university family health center
Verified Solution. Boron Family Reactions. Gas laws. Neutron to Proton Ratio. Heisenberg Uncertainty Principle. Redox Reactions. Subatomic Particles. Weak Titrate-Strong Titrant Curves. Lattice Energy. Each molecule's coefficient is represented by a variable like x, y, z , and a series of equations are set up based on the number of each type of atom. Coordination Complexes NEW. Find more answers Ask your question. Main Group Elements: Periodic Trends.
A pH of 7 means a solution is neutral. Anything below pH 7 is an acid, and anything above pH 7 is an alkali. Watch this video to find out more about neutralisation close neutralisation A chemical reaction in which an acid reacts with a base or an alkali to form a salt and water.
Average Bond Order. Benzene Reactions. Next problem. Thermal Equilibrium. Process: Start with the most complex molecule or the one with the most elements, and adjust the coefficients of the reactants and products until the equation is balanced. Intro to Electrochemical Cells. Dimensional Analysis. Hydrogen Isotopes. Millikan Oil Drop Experiment. Ester Reactions: Esterification.

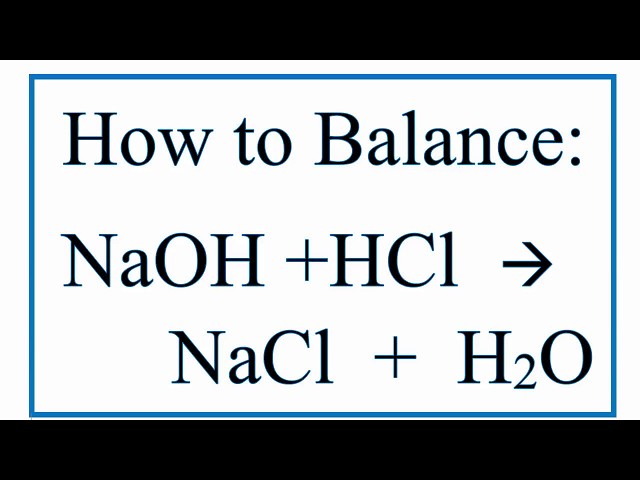
0 thoughts on “Balanced equation for hydrochloric acid and sodium hydroxide”