Are ionic bonds stronger than covalent bonds
Byju's Answer. Which bond is stronger- ionic or covalent? Open in App. Chemical bonds can be either formed by sharing of electrons or by transfer of electrons, by which the bonding atoms attain an octet[or duplet] state.
Atoms separated by a great distance cannot link; rather, they must come close enough for the electrons in their valence shells to interact. But do atoms ever actually touch one another? Most physicists would say no, because the negatively charged electrons in their valence shells repel one another. No force within the human body—or anywhere in the natural world—is strong enough to overcome this electrical repulsion. So when you read about atoms linking together or colliding, bear in mind that the atoms are not merging in a physical sense. Instead, atoms link by forming a chemical bond. A bond is a weak or strong electrical attraction that holds atoms in the same vicinity.
Are ionic bonds stronger than covalent bonds
Post by Jessica Castellanos » Tue Nov 19, am. Post by jisulee1C » Tue Nov 19, am. Post by joshtully » Mon Oct 26, am. Post by isha dis3d » Wed Oct 28, pm. Post by David Y » Sun Nov 01, am. Laurence Lavelle Skip to content. Quick links. Email Link. Is ionic or covalent stronger? I was always taught throughout high school that covalent bonds are stronger than ionic bonds. But I came across a question in the textbook asking which of two substances would have a higher boiling point and I know that it has to be the one with the stronger bond. One of them is covalent HCl and the other is ionic NaCl. I googled which was stronger and I found some people saying ionic is stronger and some people saying covalent is stronger.
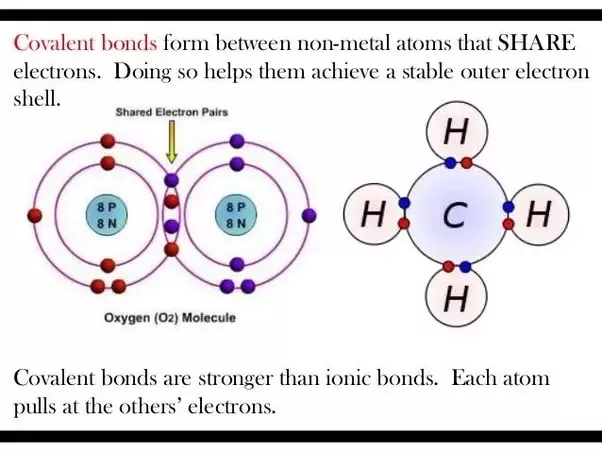
Water molecules also strongly attract other types of charged molecules as well as ions. For the ionic solid MXthe lattice energy is the enthalpy change of the process:. Because of the close sharing of pairs of electrons one electron from each of two atomscovalent bonds are stronger than ionic bonds.
In this section, you will learn about the bond strength of covalent bonds, and then compare that to the strength of ionic bonds, which is related to the lattice energy of a compound. Stable molecules exist because covalent bonds hold the atoms together. We measure the strength of a covalent bond by the energy required to break it, that is, the energy necessary to separate the bonded atoms. Separating any pair of bonded atoms requires energy; the stronger a bond, the greater the energy required to break it. The energy required to break a specific covalent bond in one mole of gaseous molecules is called the bond energy or the bond dissociation energy. Breaking a bond always require energy to be added to the molecule.
In this section, you will learn about the bond strength of covalent bonds, and then compare that to the strength of ionic bonds, which is related to the lattice energy of a compound. Stable molecules exist because covalent bonds hold the atoms together. We measure the strength of a covalent bond by the energy required to break it, that is, the energy necessary to separate the bonded atoms. Separating any pair of bonded atoms requires energy. The stronger a bond, the greater the energy required to break it. The energy required to break a specific covalent bond in one mole of gaseous molecules is called the bond energy or the bond dissociation energy. Molecules with three or more atoms have two or more bonds. The sum of all bond energies in such a molecule is equal to the standard enthalpy change for the endothermic reaction that breaks all the bonds in the molecule. The strength of a bond between two atoms increases as the number of electron pairs in the bond increases.
Are ionic bonds stronger than covalent bonds
In this section, you will learn about the bond strength of covalent bonds, and then compare that to the strength of ionic bonds, which is related to the lattice energy of a compound. Stable molecules exist because covalent bonds hold the atoms together. We measure the strength of a covalent bond by the energy required to break it, that is, the energy necessary to separate the bonded atoms.
0405 phone number
Its atomic number is nine, and it has seven electrons in its valence shell. One of them is covalent HCl and the other is ionic NaCl. I would assume that ionic would be the strongest because the atoms actually give up or gain electrons, they don't share them as is the case with covalent bonds. This is my understanding, I hope that helps! Recall that an atom typically has the same number of positively charged protons and negatively charged electrons. A carbon atom has four electrons in its valence shell. ZnO would have the larger lattice energy because the Z values of both the cation and the anion in ZnO are greater, and the interionic distance of ZnO is smaller than that of NaCl. Why was the water alone not effective in cleaning the bowl? Covalent bonds are created by electronegativity and tend to be weaker. Twice that value is —
In this section, you will learn about the bond strength of covalent bonds, and then compare that to the strength of ionic bonds, which is related to the lattice energy of a compound.
Converting one mole of fluorine atoms into fluoride ions is an exothermic process, so this step gives off energy the electron affinity and is shown as decreasing along the y -axis. Standard IX Chemistry. According to the octet rule, it will readily participate in chemical reactions that result in its valence shell having eight electrons. In chemistry, ionics bonds are usually considered stronger as we work with ionic bonds in the solid state more often while in biology, ionic bonds are considered weaker as molecules tend to be in solution. In the next step, we account for the energy required to break the F—F bond to produce fluorine atoms. Because every proton exerts an identical positive charge, a nucleus that contains eight protons exerts a charge eight times greater than a nucleus that contains one proton. When ionic bonds are in water they are much weaker. It can be obtained by the fermentation of sugar or synthesized by the hydration of ethylene in the following reaction: A set of Lewis structures show a chemical reaction. For ionic compounds, lattice energies are associated with many interactions, as cations and anions pack together in an extended lattice. Hydrogen bonding occurs because the weakly negative oxygen atom in one water molecule is attracted to the weakly positive hydrogen atoms of two other water molecules [link]. For the ionic solid MX , the lattice energy is the enthalpy change of the process:. Hydrogen bonds are relatively weak, and therefore are indicated with a dotted rather than a solid line.

Not to tell it is more.