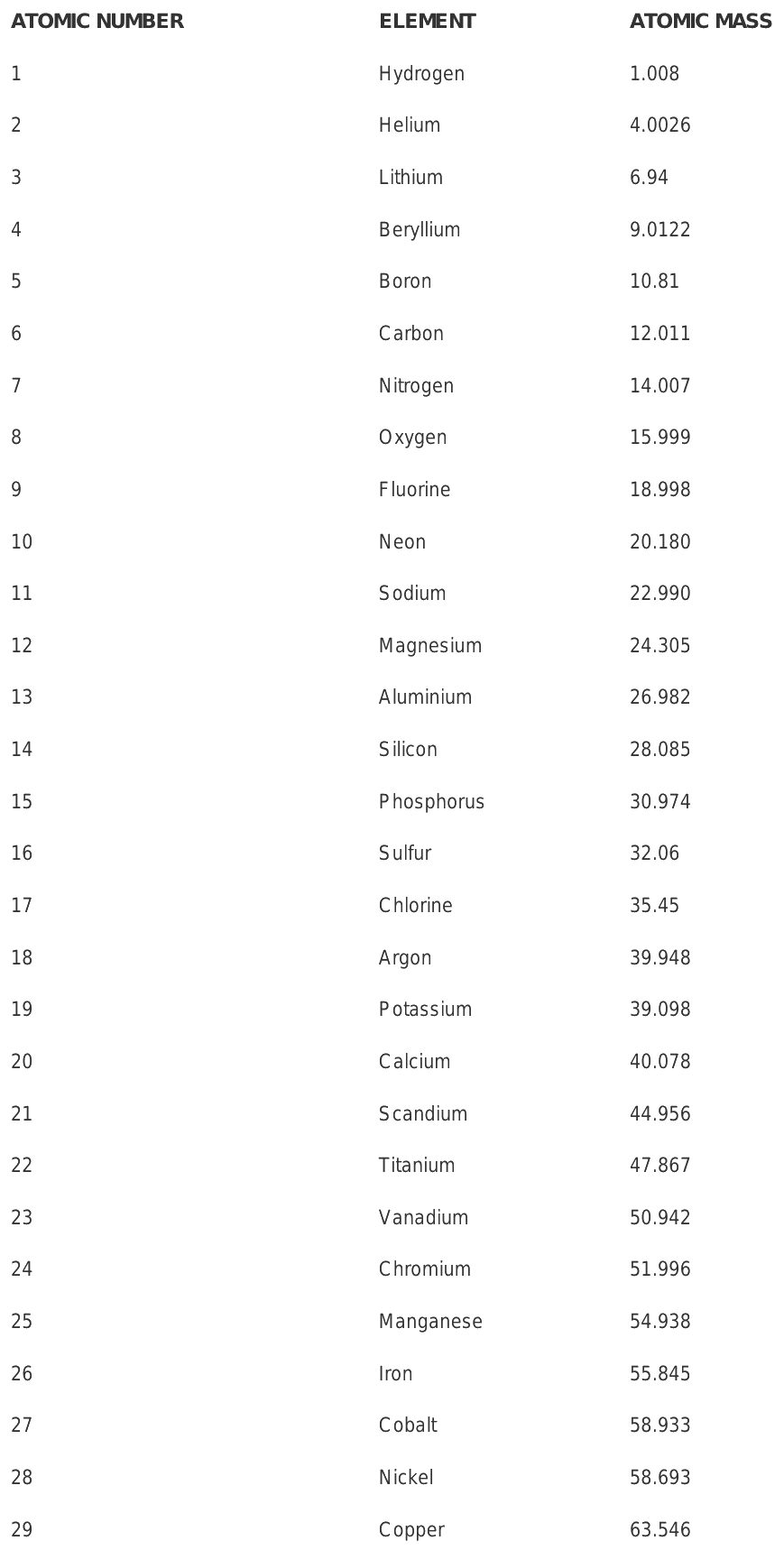
Approx atomic mass of first 30 elements
The atomic mass of elements is measured with the help of unified atomic mass units. One unified atomic mass unit can be quantified as the weight of one-twelfth of the mass of a carbon atom considering that it is at rest.
Open navigation menu. Close suggestions Search Search. User Settings. Skip carousel. Carousel Previous. Carousel Next.
Approx atomic mass of first 30 elements
Atomic mass is the total mass of all subatomic particles of an atom, including protons, neutrons, and electrons. One dalton is equivalent to one-twelfth of the mass of a carbon atom at rest in its ground state. This definition provides a standard reference point for measuring atomic masses. The atomic mass of an individual atom is closely related to its mass number, which represents the total number of protons and neutrons in the nucleus. This relationship helps simplify calculations and understanding of atomic masses. Atomic Mass of an element is a measure of the average mass of its atoms. Atomic mass of an element is defined as the total mass of one atom of that element. The atomic number can also be called atomic weight. An atomic mass unit amu is a unit of mass used to express atomic and molecular mass. It is equivalent to approximately 1.
This was the complete discussion on atomic mass, its calculation and the difference between atomic mass and number.
The atomic mass in Chemistry is the average mass of the atoms of an element measured in atomic mass units amu. The atomic mass is simply defined as the weighted average of all of the isotopes of an element, in which the mass of each isotope is multiplied by the abundance of that particular isotope. An interesting point to note is that it is also referred to as atomic weight. In this article, we will learn about the following things: the atomic mass of elements in detail, what is the atomic mass of all elements, and what is the atomic number and atomic mass of elements. Since we have seen the definition of atomic mass let us discuss it in detail. The atomic mass of a solitary atom is its absolute mass and is regularly expressed in atomic mass units or amu. For example, a normal carbon atom with six neutrons and six protons is denoted as carbon
As early chemists worked to purify ores and discovered more elements, they realized that various elements could be grouped together by their similar chemical behaviors. One such grouping includes lithium Li , sodium Na , and potassium K : These elements all are shiny, conduct heat and electricity well, and have similar chemical properties. A second grouping includes calcium Ca , strontium Sr , and barium Ba , which also are shiny, good conductors of heat and electricity, and have chemical properties in common. However, the specific properties of these two groupings are notably different from each other. For example: Li, Na, and K are much more reactive than are Ca, Sr, and Ba; Li, Na, and K form compounds with oxygen in a ratio of two of their atoms to one oxygen atom, whereas Ca, Sr, and Ba form compounds with one of their atoms to one oxygen atom. Fluorine F , chlorine Cl , bromine Br , and iodine I also exhibit similar properties to each other, but these properties are drastically different from those of any of the elements above.
Approx atomic mass of first 30 elements
Atomic mass is the total mass of all subatomic particles of an atom, including protons, neutrons, and electrons. One dalton is equivalent to one-twelfth of the mass of a carbon atom at rest in its ground state. This definition provides a standard reference point for measuring atomic masses. The atomic mass of an individual atom is closely related to its mass number, which represents the total number of protons and neutrons in the nucleus.
Katie cassidy nue
The atomic mass unit is the full form of amu. Engineering Exam Experiences. Given below is a table that lists the first 30 elements based on atomic number and their corresponding atomic mass. What is Plasma and Bose-Einstein Condensate? This article is being improved by another user right now. Atoms and Molecules. The atomic mass of oxygen is approximately Regardless of the presence of neutrons, elements are classified according to the number of protons in their nuclei. The atomic mass is determined by averaging the weight of all the isotopes of the element. Put your understanding of this concept to test by answering a few MCQs.
If you're seeing this message, it means we're having trouble loading external resources on our website.
What is atomic mass? Related Articles. View More. What is the Atomic Mass of Lithium? The overall atomic masses that are given in periodic tables like the one for hydrogen are determined for the naturally occurring isotopes of each element, weighted by the weight of those particular isotopes on earth. Mough aondofa January 17, at pm. Like Article. Atomic Mass. Culture Documents. The mass a molecule carries is known as its molecular mass, additionally known as molecular weight. Unit 2 Lesson 2 Unit 2 Lesson 2.

0 thoughts on “Approx atomic mass of first 30 elements”