Ammonia lewis dot structure
If you plan to view the video on ammonia lewis dot structure cell phone, consider your data plan and whether you should wait until you have a WiFi connection to avoid cellular charges. How to draw the Lewis dot structure for H 2 0? We know that it would have to be H-O-H because hydrogen can only form one bond. Hydrogen is always on the outside of a molecule.
Ammonia is an inorganic chemical with the chemical formula NH3. This compound is a colorless, pungent gas made of nitrogen and Hydrogen. It occurs in nature and is primarily produced by the anaerobic decay of plant and animal matter, and it has also been detected in outer space. Ammonia is directly or indirectly the precursor to most nitrogen-containing compounds. Virtually all synthetic nitrogen compounds are derived from ammonia. It is essential to understand the reaction properties of ammonia. Hence, determining the Lewis structure for the NH3 is essential.
Ammonia lewis dot structure
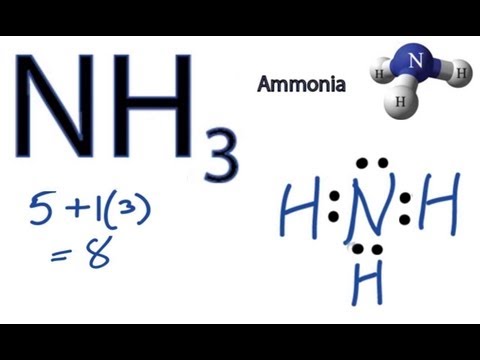
Transcript: OK, this is Dr. We're going to do the Lewis structure for NH3: ammonia or Nitrogen trihydride. On the periodic table, Nitrogen is in group 5 or 15 so it has 5 valence electrons, and then Hydrogen is in group 1. It has one valence electron, but we have 3 Hydrogens, so let's mutiply that by 3. Five plus 3, a total of 8 valence electrons. Hydrogen always goes on the outside, so let's put our Nitrogen right here, and let's put some Hydrogens around it. We have three of them; there they go, 1, 2, 3. And now we have those 8 valence electrons. We're going to form chemical bonds with those. So we'll put them between atoms first. Hydrogen only needs 2 valence electrons to have a full outer shell, so Hydrogens are going to be full with 2 valence electrons. So we have 2, 4, 6, and we have 8 total, let's just put those up here. And now, if you take a look, we can see that Nitrogen has 8 valence electrons, its octet is full; and each of the Hydrogens, each one of those has 2 valence electrons. So we're good. That's the Lewis structure for NH3.
This is Dr. So we have 2, 4, 6, and we have 8 total, let's just put those up here. This was re-drawn for neatness sake in the bottom drawing.
.
In the lewis structure of ammonia NH 3 , there are three N-H bonds and one lone pair on nitrogen atom. Lewis structure of NH 3 can be drawn by starting from valence electrons of nitrogen and hydrogen atoms in several steps. Each step of drawing the lewis structure of NH 3 is explained in detail in this tutorial. After drawing the lewis structure of NH 3 , you can decide shape of the NH 3 molecule. In the lewis structure of NH 3 , there are three N-H bonds and one lone pair on nitrogen atom. There are no lone pairs on hydrogen atoms which cannot keep more than two electrons. You have to follow several steps to draw the lewis structure of NH 3.
Ammonia lewis dot structure
Ammonia is the simplest binary hydride made up of nitrogen and hydrogen denoted by its chemical formulae as NH3. It is a stable pnictogen hydride where all the atoms are covalently bonded to achieve a reactive state. Ammonia is lighter than the air, colorless, and pungent in smell. It is a common nitrogenous waste of aquatic animals and an essential composition of the nutritional needs of terrestrial animals.
Soursop perth
Ammonia, a colorless gas with a distinct odor, is a building-block chemical and a key component in the manufacture of many products people use every day. Ammonia: define and importantly Ammonia is an inorganic chemical with the chemical formula NH3. To calculate the formal charge on an atom. Transcript: OK, this is Dr. Oxygen has 6 valence electrons. Virtually all synthetic nitrogen compounds are derived from ammonia. Like Loading It is generated in nature primarily by the decay of plant and animal matter and is used as an essential nutrient by al. So we'll put them between atoms first. So we have 2, 4, 6, and we have 8 total, let's just put those up here. Hence, determining the Lewis structure for the NH3 is essential. Janet Gray Coonce. Health hazard of Ammonia Nov 9, Ammonia is a naturally occurring compound which is a key component of the global nitrogen cycle and all living organisms. It occurs naturally throughout the environment in the air, soil and water and in plant.
.
That's the Lewis structure for NH3. It is essential to understand the reaction properties of ammonia. Ammonia is a naturally occurring compound which is a key component of the global nitrogen cycle and all living organisms. How to draw the Lewis dot structure for H 2 0? Nitrogen now with its full octet of valence electrons is isoelectric with the noble gas neon. On the periodic table, Nitrogen is in group 5 or 15 so it has 5 valence electrons, and then Hydrogen is in group 1. It is generated in nature primarily by the decay of plant and animal matter and is used as an essential nutrient by al. Opens New Window. We're going to do the Lewis structure for NH3: ammonia or Nitrogen trihydride. Remember, too, that hydrogen only needs two valence electrons to have a full outer shell. It's not particularly difficult but is an important structure.

Yes, really. And I have faced it. We can communicate on this theme. Here or in PM.
In it something is also to me it seems it is excellent idea. I agree with you.