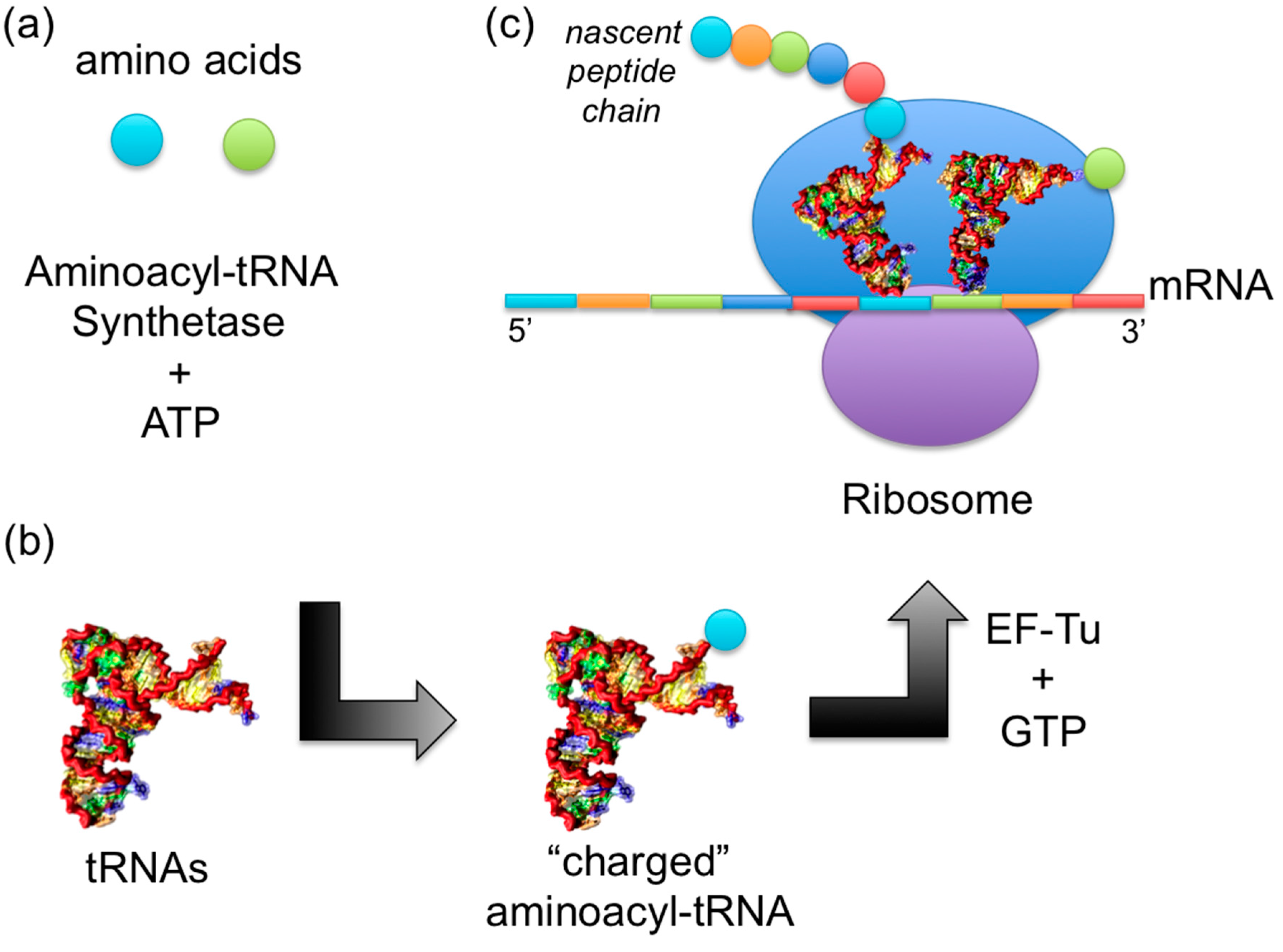
Aminoacyl-trna
It does so by catalyzing the transesterification of a specific cognate amino acid or its precursor to one of all its compatible cognate tRNAs to aminoacyl-trna an aminoacyl-tRNA, aminoacyl-trna.
Past events. These enzymes are not gentle with tRNA molecules. The enzyme shown in red firmly grips the anticodon loop shown in yellow , spreading the three bases widely apart for better recognition. At the other end, the enzyme unpairs one base at the beginning of the chain, seen curving upward here, and kinks the long acceptor end of the chain into a tight hairpin, seen here curving downward. This places the 2' hydroxyl on the last nucleotide in the active site, where ATP colored white and the amino acid not present in this structure are bound. Select the JSmol tab to explore these structures in an interactive view.
Aminoacyl-trna
Federal government websites often end in. The site is secure. The aminoacyl-tRNA synthetases are an essential and universally distributed family of enzymes that plays a critical role in protein synthesis, pairing tRNAs with their cognate amino acids for decoding mRNAs according to the genetic code. Synthetases help to ensure accurate translation of the genetic code by using both highly accurate cognate substrate recognition and stringent proofreading of noncognate products. While alterations in the quality control mechanisms of synthetases are generally detrimental to cellular viability, recent studies suggest that in some instances such changes facilitate adaption to stress conditions. Beyond their central role in translation, synthetases are also emerging as key players in an increasing number of other cellular processes, with far-reaching consequences in health and disease. The biochemical versatility of the synthetases has also proven pivotal in efforts to expand the genetic code, further emphasizing the wide-ranging roles of the aminoacyl-tRNA synthetase family in synthetic and natural biology. The product of this reaction, an aminoacyl-tRNA aa-tRNA , is delivered by elongation factors to the ribosome to take part in protein synthesis. The discovery of the aaRSs and their role in protein synthesis began in the 50s and 60s when it was reported that amino acids were required to undergo an activation process in order to take part in protein synthesis Hoagland ; Zamecnik et al. The discovery of tRNA Hoagland et al. AaRSs fulfill two extremely important roles in translation: not only do they provide the building blocks for protein synthesis, they are also the only enzymes capable of implementing the genetic code Woese et al. A total of 23 aaRSs have been described so far, one for each of the 20 proteinogenic amino acids except for lysine, for which there are two plus pyrrolysyl-tRNA synthetase PylRS and phosphoseryl-tRNA synthetase SepRS , enzymes with a more restricted distribution that are only found in some bacterial and archaeal genomes Cusack et al. It is also worth noting that in eukaryotes the protein synthesis machineries of mitochondria and chloroplasts generally utilize their own, bacterial-like sets of synthetases and tRNAs that are distinct from their cytosolic counterparts Tzagoloff et al. In the second part of the reaction, the hydroxyl group of the adenine 76 nt attacks the carbonyl carbon of the adenylate, forming aminoacyl-tRNA and AMP Fig. While the two-step aminoacylation reaction is universally conserved, the aaRSs that catalyze it show extensive structural, and in some instances functional, diversity as described in detail below.
Corresponding author: ude. Selenocysteine was the first noncanonical amino acid discovered aminoacyl-trna the original 20 amino acids of the genetic code Cone et al. Separation, aminoacyl-trna, properties, and stimulation of adenosine triphosphate-pyrophosphate exchange aminoacyl-trna acceptor ribonucleic acid.
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. This typical function has been well recognized over the past few decades.
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. This typical function has been well recognized over the past few decades. However, accumulating evidence reveals that ARSs are involved in a wide range of physiological and pathological processes apart from translation.
Aminoacyl-trna
Federal government websites often end in. The site is secure. The aminoacyl-tRNA synthetases are prominently known for their classic function in the first step of protein synthesis, where they bear the responsibility of setting the genetic code. Each enzyme is exquisitely adapted to covalently link a single standard amino acid to its cognate set of tRNA isoacceptors. These ancient enzymes have evolved idiosyncratically to host alternate activities that go far beyond their aminoacylation role and impact a wide range of other metabolic pathways and cell signaling processes. The family of aminoacyl-tRNA synthetases have also been suggested as a remarkable scaffold to incorporate new domains that would drive evolution and the emergence of new organisms with more complex function. Because they are essential, the tRNA synthetases have served as pharmaceutical targets for drug and antibiotic development. The recent unfolding of novel important functions for this family of proteins offers new and promising pathways for therapeutic development to treat diverse human diseases. The aminoacyl-tRNA synthetases aaRSs comprise an ancient family of enzymes that are responsible for the first step of protein synthesis. This diverse set of proteins is united by a common aminoacylation reaction, which attaches an amino acid to its cognate tRNA.
Derailed thesaurus
Acta Crystallograph. Structures for both forms have been resolved and shown to use similar mechanisms for substrate recognition and even recognize the same tRNA determinants Terada et al. Alternative stable conformation capable of protein misinteraction links tRNA synthetase to peripheral neuropathy. Editing factors Another important component of the translation quality control machinery is the trans -editing family, freestanding proteins that are not synthetases but are in some cases homologs to the editing domains of such enzymes. Moreover, thiazolinone derivatives as WRS inhibitors showed higher activity against Gram positive bacterial strains than Gram negative bacterial strains These enzymes make about one mistake in 10, In the decades after their initial discovery, the synthases were extensively characterized and their roles in tRNA charging analyzed in great detail. AlaRS faces a unique challenge when maintaining fidelity as it is able to activate not only the smaller Gly but also the bigger Ser. Li, X. Nucleic Acids Research. More than different amino acids have been identified in naturally occurring proteins, although outside of the 20 proteinogenic ones nearly all of them are the result of post-translation modifications Uy and Wold ; Macek et al. Curr Opin Struct Biol 7 : — Specifically, these two myositis autoantigens selectively induced migration of lymphocytes, activated monocytes, and immature DCs. Exploiting the difference between tRNA identity elements from different species represented the first successful attempt to expand the genetic code with TyrRS. Notably, glutamyl-prolyl-tRNA synthetase EPRS can catalyze the charging for glutamic acid and proline in the cytoplasm, therefore a full set of ARSs responsible for the charging of the 20 canonical amino acids exists in both the cytoplasm and mitochondria.
The aminoacyl-tRNA synthetases are an essential and universally distributed family of enzymes that plays a critical role in protein synthesis, pairing tRNAs with their cognate amino acids for decoding mRNAs according to the genetic code. Synthetases help to ensure accurate translation of the genetic code by using both highly accurate cognate substrate recognition and stringent proofreading of noncognate products. While alterations in the quality control mechanisms of synthetases are generally detrimental to cellular viability, recent studies suggest that in some instances such changes facilitate adaption to stress conditions.
This is possible if the tRNA determinants for the donor and receiving species do not match. PMID Curr Opin Chem Biol 46 : — Editing function of Escherichia coli cysteinyl-tRNA synthetase: cyclization of cysteine to cysteine thiolactone. In many instances, the high specificity of the active site is enough to circumvent the need for proofreading and editing. The natural product borrelidin was a threonyl-tRNA synthetase ThrRS inhibitor with various biological functions such as antifungal, antibacterial, antimalarial, and antiangiogenic activities Rajendran, V. Disrupted function and axonal distribution of mutant tyrosyl-tRNA synthetase in dominant intermediate Charcot-Marie-Tooth neuropathy. Nucleic Acids Res 47 : — Baddal, B. Probing the principles of amino acid selection using the alanyl-tRNA synthetase from Escherichia coli. Coding from a distance: dissection of the mRNA determinants required.

I am sorry, it does not approach me. There are other variants?
Excuse, that I interfere, but it is necessary for me little bit more information.
It be no point.